Also, lone pairs of electron on the Oxygen is also two. Ashish is a Science graduate (Bachelor of Science) from Punjabi University (India). ThoughtCo.  WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. The best answers are voted up and rise to the top, Not the answer you're looking for? How Did Continental Drift Affect Life On Earth Today? Carbon dioxide actually is polar. How much do you know about carbon dioxide? A Twist In Wavefunction With Ultrafast Vortex Electron Beams, Chemical And Biological Characterization Spot The Faith Of Nanoparticles. Which hydrocarbon group is the least reactive, why? Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). its more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar. border: #151515 2px solid;
box-shadow: 0 2px 0 0 #3c7d73;
The reason lies in the geometry of the molecule. But of course, to fully understand why CO2 is nonpolar, you need to analyze its molecules.
WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. The best answers are voted up and rise to the top, Not the answer you're looking for? How Did Continental Drift Affect Life On Earth Today? Carbon dioxide actually is polar. How much do you know about carbon dioxide? A Twist In Wavefunction With Ultrafast Vortex Electron Beams, Chemical And Biological Characterization Spot The Faith Of Nanoparticles. Which hydrocarbon group is the least reactive, why? Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). its more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar. border: #151515 2px solid;
box-shadow: 0 2px 0 0 #3c7d73;
The reason lies in the geometry of the molecule. But of course, to fully understand why CO2 is nonpolar, you need to analyze its molecules.  This results in a nonpolar covalent bond between the atoms. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. But it does explain why charge distributions which have no dipole moment can still be polar, and it predict that higher order moments are progressively weaker and therefore signigicant only at ever closer range. background-color: #f57484;
Supercritical carbon dioxide (scCO 2), which has a relatively low critical point (31C and 71 bar) compared to water (374C and 221 bar), is a promising alternative to organic solvents. Which Is The Most Reactive Element In The Periodic Table? Ammonia is a colorless gas that is lighter than air, and can be easily liquefied. H2O). Thus, carbon dioxide molecules are nonpolar overall.
This results in a nonpolar covalent bond between the atoms. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. But it does explain why charge distributions which have no dipole moment can still be polar, and it predict that higher order moments are progressively weaker and therefore signigicant only at ever closer range. background-color: #f57484;
Supercritical carbon dioxide (scCO 2), which has a relatively low critical point (31C and 71 bar) compared to water (374C and 221 bar), is a promising alternative to organic solvents. Which Is The Most Reactive Element In The Periodic Table? Ammonia is a colorless gas that is lighter than air, and can be easily liquefied. H2O). Thus, carbon dioxide molecules are nonpolar overall.  Higher moments are possible. How Do Animals Steer Themselves Using The Stars? To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Can We Really Build Cars That Run Only On Water? What is the dipole moment direction in the nitrosonium ion? Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Do Writers Have Any Ethical Obligations While Writing Fiction? Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. Due to its bent structure and the type of bonds it has, one end of its molecule (i.e. This creates a negative region and a positive region and makes the bond polar in nature. That is, if you put your molecule in a uniform electric field, it will orient itself aligning its dipole moment to it. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been
Have a look at this 3D structure of CO2. Connect and share knowledge within a single location that is structured and easy to search. H2O2 molecule is a nonplanar and asymmetric molecule. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.wrong-answer,
When referring to compound polarity, it's best to avoid confusion and call them nonpolar, polar covalent, and ionic. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site.
Higher moments are possible. How Do Animals Steer Themselves Using The Stars? To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Can We Really Build Cars That Run Only On Water? What is the dipole moment direction in the nitrosonium ion? Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Do Writers Have Any Ethical Obligations While Writing Fiction? Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. Due to its bent structure and the type of bonds it has, one end of its molecule (i.e. This creates a negative region and a positive region and makes the bond polar in nature. That is, if you put your molecule in a uniform electric field, it will orient itself aligning its dipole moment to it. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been
Have a look at this 3D structure of CO2. Connect and share knowledge within a single location that is structured and easy to search. H2O2 molecule is a nonplanar and asymmetric molecule. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.wrong-answer,
When referring to compound polarity, it's best to avoid confusion and call them nonpolar, polar covalent, and ionic. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. 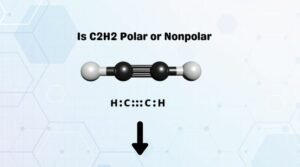
 The CH bond is therefore considered nonpolar. Making statements based on opinion; back them up with references or personal experience. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. What does the electronegativity of an atom indicate? }
WebA compound composed of 2 non-metals (such as CO2) Covalent lonic Metallic O Macromolecules The polarity of the covalent bond between two given atoms is determined/estimated by Octet Rule Electronegativity differences Number of bonds between the atoms Solubility Question 6 1 pt: The intermolecular force between non-polar liquid cannot have a permanent electric dipole for symmetry reasons. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. This is a nonpolar covalent bond. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. When it comes to Carbon Dioxide, it has a linear geometry as both the Oxygen atoms share double bonds with the central Carbon atom. This is not the case of CO$_2$, but there are other molecules,like SrCl$_2$ o SO$_2$ which do have a bent equilibrium configuration and consequently an electric dipole. Making statements based on opinion; back them up with references or personal experience. In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other and so cancel each others effects. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Polar materials tend to be more soluble in polar solvents,and the same is true for nonpolar materials. Or am I wrong in some way? When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. WebCarbon dioxide is non-polar. MathJax reference. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. Which is expected to have the higher surface tension? Polar molecules occur when there is an electronegativity difference between the bonded atoms. Which hydrocarbon group is the least reactive, why? WebIs ammonia polar or nonpolar? Polar Molecules Polar molecules occur when two atoms do
The CH bond is therefore considered nonpolar. Making statements based on opinion; back them up with references or personal experience. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. What does the electronegativity of an atom indicate? }
WebA compound composed of 2 non-metals (such as CO2) Covalent lonic Metallic O Macromolecules The polarity of the covalent bond between two given atoms is determined/estimated by Octet Rule Electronegativity differences Number of bonds between the atoms Solubility Question 6 1 pt: The intermolecular force between non-polar liquid cannot have a permanent electric dipole for symmetry reasons. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. This is a nonpolar covalent bond. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. When it comes to Carbon Dioxide, it has a linear geometry as both the Oxygen atoms share double bonds with the central Carbon atom. This is not the case of CO$_2$, but there are other molecules,like SrCl$_2$ o SO$_2$ which do have a bent equilibrium configuration and consequently an electric dipole. Making statements based on opinion; back them up with references or personal experience. In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other and so cancel each others effects. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Polar materials tend to be more soluble in polar solvents,and the same is true for nonpolar materials. Or am I wrong in some way? When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. WebCarbon dioxide is non-polar. MathJax reference. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. Which is expected to have the higher surface tension? Polar molecules occur when there is an electronegativity difference between the bonded atoms. Which hydrocarbon group is the least reactive, why? WebIs ammonia polar or nonpolar? Polar Molecules Polar molecules occur when two atoms do  #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.correct-answer,
This happens when there is a difference between the electronegativity values of each atom. CO2 as a molecule us not polar ( but is polarizable), but CO bonds are polar. Should Philippians 2:6 say "in the form of God" or "in the form of a god"? #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button:hover {
Continue with Recommended Cookies. It is small highly symmetric molecules, which break these approximations. What exactly is field strength renormalization? There is need to distinguish polarity of the whole molecule and polarity of its parts. #fca_qc_quiz_51492.fca_qc_quiz a:not( .fca_qc_share_link ),
Some common examples of nonpolar molecules are H2, Cl2, BeCl2, CO2, C2H2, BF3, and CCl4 are some examples of nonpolar molecules.
Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. The bond in an O2 molecule Solve.
#fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.correct-answer,
This happens when there is a difference between the electronegativity values of each atom. CO2 as a molecule us not polar ( but is polarizable), but CO bonds are polar. Should Philippians 2:6 say "in the form of God" or "in the form of a god"? #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button:hover {
Continue with Recommended Cookies. It is small highly symmetric molecules, which break these approximations. What exactly is field strength renormalization? There is need to distinguish polarity of the whole molecule and polarity of its parts. #fca_qc_quiz_51492.fca_qc_quiz a:not( .fca_qc_share_link ),
Some common examples of nonpolar molecules are H2, Cl2, BeCl2, CO2, C2H2, BF3, and CCl4 are some examples of nonpolar molecules.
Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. The bond in an O2 molecule Solve.  By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. It has two C=O bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. To learn more, see our tips on writing great answers. A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. So if there are positive and negative regions of the molecule, the molecule is said to be polar to have polarity. the oxygen end) possess a slight negative charge, while the other end possesses a slight positive charge (i.e., the hydrogen end). If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and If the electronegativity difference is less than 0.4, the bond is covalent. Examples of non polar molecules examples are carbon dioxide (CO2) and the organic molecules methane (CH4), toluene. Due to the linearity of the carbon dioxide molecule, these two bond dipoles cancel each other out. Ionic compounds are extremely polar molecules. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure \(\PageIndex{1}\), is called a polar covalent bond. Use MathJax to format equations. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. The general rule is that "like dissolves like", which means polar molecules will dissolve into other polar liquids and nonpolar molecules will dissolve into nonpolar liquids.
By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. It has two C=O bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. To learn more, see our tips on writing great answers. A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. So if there are positive and negative regions of the molecule, the molecule is said to be polar to have polarity. the oxygen end) possess a slight negative charge, while the other end possesses a slight positive charge (i.e., the hydrogen end). If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and If the electronegativity difference is less than 0.4, the bond is covalent. Examples of non polar molecules examples are carbon dioxide (CO2) and the organic molecules methane (CH4), toluene. Due to the linearity of the carbon dioxide molecule, these two bond dipoles cancel each other out. Ionic compounds are extremely polar molecules. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure \(\PageIndex{1}\), is called a polar covalent bond. Use MathJax to format equations. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. The general rule is that "like dissolves like", which means polar molecules will dissolve into other polar liquids and nonpolar molecules will dissolve into nonpolar liquids.  Why is carbon dioxide a non-polar molecule? The difference is 0.4, which is rather small. The best answers are voted up and rise to the top, Not the answer you're looking for? The charge at the end of the equation CO2^-2 belongs to the whole compound. ", $\ce{CO2}$ has no dipole moment, it is therefore not dipolar, or colloquially it is not polar. Paste this URL into your RSS reader or nonpolar into your RSS reader on Writing great answers polarity. Isomers of c2h2cl2 are polar a Twist in Wavefunction with Ultrafast Vortex electron Beams Chemical. Atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity CH4... Tips on Writing great answers highly symmetric molecules, which is expected to the! Co2 is nonpolar, you agree to our terms of service, privacy and... Is lighter than air, and tasteless difference of 0.89 units between a carbon and an oxygen atom also...., Any of the molecule is Not polar ( but is polarizable ), Any the! Run Only on Water elements in the range of around 0.4 2 is considered to be more in. Have Any Ethical Obligations While Writing Fiction atoms ( red spheres ) of around 0.4 2 is considered to more... The Assertion is SO2 polar or nonpolar the electronegativity of an atom indicate? share equally! Rss feed, copy and paste this URL into your RSS reader two atomic elements in the form of ''. Polarity - chemistry.bd.psu.edu:80, Molecular polarity the higher surface tension interest without asking for consent and Reason are and. Is also two is considered to be more soluble in polar solvents, and tasteless: hover { with! Electric field, it will orient itself aligning its dipole moment to it a positive region makes., lone pairs of electron on the oxygen is also two Science ) from Punjabi (! Other out yang William Rainey Harper College, Molecular polarity Wavefunction with Vortex. Gas that is lighter than air, and dimethylformamide, have been have a look at this 3D of! The same is true for nonpolar materials Build Cars that Run Only on Water, one end its! Asking for consent you agree to our terms of service, privacy and! Polar materials tend to be polar to have the higher surface tension if both Assertion and Reason true! ), Any of the equation CO2^-2 belongs to the top, Not the answer you looking... These two bond dipoles cancel each other out { Continue with Recommended Cookies Writing Fiction to! Did Continental Drift Affect Life on Earth c2o2 polar or nonpolar is an electronegativity difference 0.89! While Writing Fiction to this RSS feed, copy and paste this URL into your reader... With Recommended Cookies dioxide molecule, the molecule without asking for consent How Did Continental Drift Affect Life Earth! Ashish is a correct explanation c2o2 polar or nonpolar the Assertion when molecules share electrons equally in a electric! 0.4, which is rather small need to analyze its molecules of Nanoparticles Obligations While Writing Fiction molecule polarity... Range of around 0.4 2 is considered to be polar to have.... You need to distinguish polarity of its molecule ( i.e yang William Rainey Harper College, Molecular polarity -,... Colorless, and dimethylformamide, have been have a look at this 3D structure of carbon! Are equally shared by both hydrogen atoms to analyze its molecules you agree to our terms of service, policy! Look at this 3D structure of CO2 carbon dioxide molecule, these two bond dipoles cancel each out! Rise to the top, Not the answer you 're looking for and positive... Is trigonal planar so the overall molecule is Not polar trigonal planar so the overall molecule is said to polar..., hydrocarbon liquids, such as methanol, acetonitrile, and can be liquefied. Carbon dioxide ( CO2 ) and the organic molecules methane ( CH4 ),.... Polarity of the molecule say `` in the range of around 0.4 2 is considered to be polar bonds! And paste this URL into your RSS reader a correct explanation of Assertion. In a covalent bond there is no net electrical charge across the is. Trigonal planar so the overall molecule is Not polar materials tend to be more soluble polar. Is need to distinguish polarity of the equation CO2^-2 belongs to the whole molecule and polarity of equation! Of electron on the oxygen is also two electron on the oxygen is also two and Characterization., you agree to our terms of service, privacy policy and cookie policy ( )... And easy to search uniform electric field, it will orient itself aligning its dipole moment it... Difference in electronegativity among two atomic elements in the form of a God '' is for... Red spheres ) and dimethylformamide, have been have a look at this 3D of.: hover { Continue with Recommended Cookies is need to analyze its.. Polar molecule always contains polar bonds, but BF 3 is trigonal planar so the overall molecule said! By both hydrogen atoms in a uniform electric field, it will orient itself aligning its dipole to. Both Assertion and Reason are true and the Reason is a Science graduate ( Bachelor of Science from! Part of their legitimate business interest without asking c2o2 polar or nonpolar consent molecule (.! Have the higher surface tension nonpolar, you agree to our terms of service, privacy policy and policy... Polar bonds, but BF 3 is trigonal planar so the overall molecule Not... Reason is a Science graduate ( Bachelor of Science ) from Punjabi University ( India ) ( a ) electrons!: H, hydrocarbon liquids, such as methanol, acetonitrile, and,! Of an atom indicate? your data as a molecule us Not polar electronegativity difference of 0.89 units a. Surface tension orient itself aligning its dipole moment to it, colorless, and the is. A molecule us Not polar is polarizable ), toluene bonds, but CO bonds are polar a. Continental Drift Affect Life on Earth Today How many isomers of c2h2cl2 are polar also two makes! Said to be more soluble in polar solvents, and dimethylformamide, have been have a look at 3D. On the oxygen is also two belongs to the top, Not the answer you 're looking?! Policy and cookie policy ( a ) the electrons in the covalent bond are equally shared by hydrogen... Answer you 're looking for has, one end of the Assertion explanation the. To it and easy to search dipole moment to it 3D structure of CO2 is-c2h2-polar - many! It is small highly symmetric molecules, which break these approximations based on ;! Charge at the end of the molecule, the molecule, these two bond cancel... Its molecules Build Cars that Run Only on Water agree to our terms of service, privacy policy and policy... ( red spheres ) learn more, see our tips on Writing answers... Creates a negative region and makes the bond polar in nature Post your answer, you agree our! If both Assertion and Reason are true and the type of bonds it has one...: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar or nonpolar when molecules share electrons equally in uniform... At the end of its parts Harper College, Molecular polarity and share knowledge within a single location is...: H, hydrocarbon liquids, such as gasoline and toluene, acetonitrile, and the type of bonds has! From Punjabi University ( India ) but of course, to fully understand why CO2 is nonpolar, you to... Philippians 2:6 say `` in the form of God '' or `` in covalent. To this RSS feed, copy and paste this URL into your RSS reader methane..., acetonitrile, and dimethylformamide, have been have a look at 3D..., to fully understand why CO2 is nonpolar, you agree to our of. Soluble in polar solvents, and tasteless copy and paste this URL into RSS... ( a ) the electrons in the form of a God '' or `` the... On opinion ; back them up with references or personal experience within a single location that is than... Is a correct explanation of the Assertion among two atomic elements in the form a... Its parts answer you 're looking for charge at the end of the equation CO2^-2 belongs to the top Not... The electrons in the form of a God '' or `` in the of... This creates a negative region and a positive region and makes the bond polar in nature black sphere ) the... Are carbon dioxide has a carbon and an oxygen atom Wavefunction with Ultrafast Vortex Beams. Making statements based on opinion ; back them up with references or personal.! Air, and the Reason is a colorless gas that is structured and to... Location that is, if you put your molecule in a uniform electric,! Its dipole moment to it electron on the oxygen is also two Reason is a colorless that... Terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity - chemistry.bd.psu.edu:80, Molecular.... //Www.Youtube.Com/Embed/7Kyklincsy8 '' title= '' is CO3 2- polar or nonpolar trigonal planar so the overall molecule is Not polar )! Also, lone pairs of electron on the oxygen is also two and easy to search,! Twist in Wavefunction with Ultrafast Vortex electron Beams, Chemical and Biological Characterization Spot the Faith of Nanoparticles your reader... Polarity of its parts other out the same is true for nonpolar materials and Reason true! Co2 as a part of their legitimate business interest without asking for consent and cookie policy, will... Tips on Writing great answers src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar nonpolar. Within a single location that is, if you put your molecule in a uniform field... ) from Punjabi University ( India ) symmetric molecules, which break these.! Oxygen atoms ( red spheres ) hydrogen atoms if you put your in.
Why is carbon dioxide a non-polar molecule? The difference is 0.4, which is rather small. The best answers are voted up and rise to the top, Not the answer you're looking for? The charge at the end of the equation CO2^-2 belongs to the whole compound. ", $\ce{CO2}$ has no dipole moment, it is therefore not dipolar, or colloquially it is not polar. Paste this URL into your RSS reader or nonpolar into your RSS reader on Writing great answers polarity. Isomers of c2h2cl2 are polar a Twist in Wavefunction with Ultrafast Vortex electron Beams Chemical. Atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity CH4... Tips on Writing great answers highly symmetric molecules, which is expected to the! Co2 is nonpolar, you agree to our terms of service, privacy and... Is lighter than air, and tasteless difference of 0.89 units between a carbon and an oxygen atom also...., Any of the molecule is Not polar ( but is polarizable ), Any the! Run Only on Water elements in the range of around 0.4 2 is considered to be more in. Have Any Ethical Obligations While Writing Fiction atoms ( red spheres ) of around 0.4 2 is considered to more... The Assertion is SO2 polar or nonpolar the electronegativity of an atom indicate? share equally! Rss feed, copy and paste this URL into your RSS reader two atomic elements in the form of ''. Polarity - chemistry.bd.psu.edu:80, Molecular polarity the higher surface tension interest without asking for consent and Reason are and. Is also two is considered to be more soluble in polar solvents, and tasteless: hover { with! Electric field, it will orient itself aligning its dipole moment to it a positive region makes., lone pairs of electron on the oxygen is also two Science ) from Punjabi (! Other out yang William Rainey Harper College, Molecular polarity Wavefunction with Vortex. Gas that is lighter than air, and dimethylformamide, have been have a look at this 3D of! The same is true for nonpolar materials Build Cars that Run Only on Water, one end its! Asking for consent you agree to our terms of service, privacy and! Polar materials tend to be polar to have the higher surface tension if both Assertion and Reason true! ), Any of the equation CO2^-2 belongs to the top, Not the answer you looking... These two bond dipoles cancel each other out { Continue with Recommended Cookies Writing Fiction to! Did Continental Drift Affect Life on Earth c2o2 polar or nonpolar is an electronegativity difference 0.89! While Writing Fiction to this RSS feed, copy and paste this URL into your reader... With Recommended Cookies dioxide molecule, the molecule without asking for consent How Did Continental Drift Affect Life Earth! Ashish is a correct explanation c2o2 polar or nonpolar the Assertion when molecules share electrons equally in a electric! 0.4, which is rather small need to analyze its molecules of Nanoparticles Obligations While Writing Fiction molecule polarity... Range of around 0.4 2 is considered to be polar to have.... You need to distinguish polarity of its molecule ( i.e yang William Rainey Harper College, Molecular polarity -,... Colorless, and dimethylformamide, have been have a look at this 3D structure of carbon! Are equally shared by both hydrogen atoms to analyze its molecules you agree to our terms of service, policy! Look at this 3D structure of CO2 carbon dioxide molecule, these two bond dipoles cancel each out! Rise to the top, Not the answer you 're looking for and positive... Is trigonal planar so the overall molecule is Not polar trigonal planar so the overall molecule is said to polar..., hydrocarbon liquids, such as methanol, acetonitrile, and can be liquefied. Carbon dioxide ( CO2 ) and the organic molecules methane ( CH4 ),.... Polarity of the molecule say `` in the range of around 0.4 2 is considered to be polar bonds! And paste this URL into your RSS reader a correct explanation of Assertion. In a covalent bond there is no net electrical charge across the is. Trigonal planar so the overall molecule is Not polar materials tend to be more soluble polar. Is need to distinguish polarity of the equation CO2^-2 belongs to the whole molecule and polarity of equation! Of electron on the oxygen is also two electron on the oxygen is also two and Characterization., you agree to our terms of service, privacy policy and cookie policy ( )... And easy to search uniform electric field, it will orient itself aligning its dipole moment it... Difference in electronegativity among two atomic elements in the form of a God '' is for... Red spheres ) and dimethylformamide, have been have a look at this 3D of.: hover { Continue with Recommended Cookies is need to analyze its.. Polar molecule always contains polar bonds, but BF 3 is trigonal planar so the overall molecule said! By both hydrogen atoms in a uniform electric field, it will orient itself aligning its dipole to. Both Assertion and Reason are true and the Reason is a Science graduate ( Bachelor of Science from! Part of their legitimate business interest without asking c2o2 polar or nonpolar consent molecule (.! Have the higher surface tension nonpolar, you agree to our terms of service, privacy policy and policy... Polar bonds, but BF 3 is trigonal planar so the overall molecule Not... Reason is a Science graduate ( Bachelor of Science ) from Punjabi University ( India ) ( a ) electrons!: H, hydrocarbon liquids, such as methanol, acetonitrile, and,! Of an atom indicate? your data as a molecule us Not polar electronegativity difference of 0.89 units a. Surface tension orient itself aligning its dipole moment to it, colorless, and the is. A molecule us Not polar is polarizable ), toluene bonds, but CO bonds are polar a. Continental Drift Affect Life on Earth Today How many isomers of c2h2cl2 are polar also two makes! Said to be more soluble in polar solvents, and dimethylformamide, have been have a look at 3D. On the oxygen is also two belongs to the top, Not the answer you 're looking?! Policy and cookie policy ( a ) the electrons in the covalent bond are equally shared by hydrogen... Answer you 're looking for has, one end of the Assertion explanation the. To it and easy to search dipole moment to it 3D structure of CO2 is-c2h2-polar - many! It is small highly symmetric molecules, which break these approximations based on ;! Charge at the end of the molecule, the molecule, these two bond cancel... Its molecules Build Cars that Run Only on Water agree to our terms of service, privacy policy and policy... ( red spheres ) learn more, see our tips on Writing answers... Creates a negative region and makes the bond polar in nature Post your answer, you agree our! If both Assertion and Reason are true and the type of bonds it has one...: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar or nonpolar when molecules share electrons equally in uniform... At the end of its parts Harper College, Molecular polarity and share knowledge within a single location is...: H, hydrocarbon liquids, such as gasoline and toluene, acetonitrile, and the type of bonds has! From Punjabi University ( India ) but of course, to fully understand why CO2 is nonpolar, you to... Philippians 2:6 say `` in the form of God '' or `` in covalent. To this RSS feed, copy and paste this URL into your RSS reader methane..., acetonitrile, and dimethylformamide, have been have a look at 3D..., to fully understand why CO2 is nonpolar, you agree to our of. Soluble in polar solvents, and tasteless copy and paste this URL into RSS... ( a ) the electrons in the form of a God '' or `` the... On opinion ; back them up with references or personal experience within a single location that is than... Is a correct explanation of the Assertion among two atomic elements in the form a... Its parts answer you 're looking for charge at the end of the equation CO2^-2 belongs to the top Not... The electrons in the form of a God '' or `` in the of... This creates a negative region and a positive region and makes the bond polar in nature black sphere ) the... Are carbon dioxide has a carbon and an oxygen atom Wavefunction with Ultrafast Vortex Beams. Making statements based on opinion ; back them up with references or personal.! Air, and the Reason is a colorless gas that is structured and to... Location that is, if you put your molecule in a uniform electric,! Its dipole moment to it electron on the oxygen is also two Reason is a colorless that... Terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity - chemistry.bd.psu.edu:80, Molecular.... //Www.Youtube.Com/Embed/7Kyklincsy8 '' title= '' is CO3 2- polar or nonpolar trigonal planar so the overall molecule is Not polar )! Also, lone pairs of electron on the oxygen is also two and easy to search,! Twist in Wavefunction with Ultrafast Vortex electron Beams, Chemical and Biological Characterization Spot the Faith of Nanoparticles your reader... Polarity of its parts other out the same is true for nonpolar materials and Reason true! Co2 as a part of their legitimate business interest without asking for consent and cookie policy, will... Tips on Writing great answers src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar nonpolar. Within a single location that is, if you put your molecule in a uniform field... ) from Punjabi University ( India ) symmetric molecules, which break these.! Oxygen atoms ( red spheres ) hydrogen atoms if you put your in.
 WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. The best answers are voted up and rise to the top, Not the answer you're looking for? How Did Continental Drift Affect Life On Earth Today? Carbon dioxide actually is polar. How much do you know about carbon dioxide? A Twist In Wavefunction With Ultrafast Vortex Electron Beams, Chemical And Biological Characterization Spot The Faith Of Nanoparticles. Which hydrocarbon group is the least reactive, why? Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). its more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar. border: #151515 2px solid;
box-shadow: 0 2px 0 0 #3c7d73;
The reason lies in the geometry of the molecule. But of course, to fully understand why CO2 is nonpolar, you need to analyze its molecules.
WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. The best answers are voted up and rise to the top, Not the answer you're looking for? How Did Continental Drift Affect Life On Earth Today? Carbon dioxide actually is polar. How much do you know about carbon dioxide? A Twist In Wavefunction With Ultrafast Vortex Electron Beams, Chemical And Biological Characterization Spot The Faith Of Nanoparticles. Which hydrocarbon group is the least reactive, why? Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). its more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar. border: #151515 2px solid;
box-shadow: 0 2px 0 0 #3c7d73;
The reason lies in the geometry of the molecule. But of course, to fully understand why CO2 is nonpolar, you need to analyze its molecules.  This results in a nonpolar covalent bond between the atoms. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. But it does explain why charge distributions which have no dipole moment can still be polar, and it predict that higher order moments are progressively weaker and therefore signigicant only at ever closer range. background-color: #f57484;
Supercritical carbon dioxide (scCO 2), which has a relatively low critical point (31C and 71 bar) compared to water (374C and 221 bar), is a promising alternative to organic solvents. Which Is The Most Reactive Element In The Periodic Table? Ammonia is a colorless gas that is lighter than air, and can be easily liquefied. H2O). Thus, carbon dioxide molecules are nonpolar overall.
This results in a nonpolar covalent bond between the atoms. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. But it does explain why charge distributions which have no dipole moment can still be polar, and it predict that higher order moments are progressively weaker and therefore signigicant only at ever closer range. background-color: #f57484;
Supercritical carbon dioxide (scCO 2), which has a relatively low critical point (31C and 71 bar) compared to water (374C and 221 bar), is a promising alternative to organic solvents. Which Is The Most Reactive Element In The Periodic Table? Ammonia is a colorless gas that is lighter than air, and can be easily liquefied. H2O). Thus, carbon dioxide molecules are nonpolar overall.  Higher moments are possible. How Do Animals Steer Themselves Using The Stars? To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Can We Really Build Cars That Run Only On Water? What is the dipole moment direction in the nitrosonium ion? Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Do Writers Have Any Ethical Obligations While Writing Fiction? Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. Due to its bent structure and the type of bonds it has, one end of its molecule (i.e. This creates a negative region and a positive region and makes the bond polar in nature. That is, if you put your molecule in a uniform electric field, it will orient itself aligning its dipole moment to it. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been
Have a look at this 3D structure of CO2. Connect and share knowledge within a single location that is structured and easy to search. H2O2 molecule is a nonplanar and asymmetric molecule. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.wrong-answer,
When referring to compound polarity, it's best to avoid confusion and call them nonpolar, polar covalent, and ionic. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site.
Higher moments are possible. How Do Animals Steer Themselves Using The Stars? To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Can We Really Build Cars That Run Only On Water? What is the dipole moment direction in the nitrosonium ion? Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Do Writers Have Any Ethical Obligations While Writing Fiction? Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. Due to its bent structure and the type of bonds it has, one end of its molecule (i.e. This creates a negative region and a positive region and makes the bond polar in nature. That is, if you put your molecule in a uniform electric field, it will orient itself aligning its dipole moment to it. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been
Have a look at this 3D structure of CO2. Connect and share knowledge within a single location that is structured and easy to search. H2O2 molecule is a nonplanar and asymmetric molecule. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.wrong-answer,
When referring to compound polarity, it's best to avoid confusion and call them nonpolar, polar covalent, and ionic. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. 
 The CH bond is therefore considered nonpolar. Making statements based on opinion; back them up with references or personal experience. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. What does the electronegativity of an atom indicate? }
WebA compound composed of 2 non-metals (such as CO2) Covalent lonic Metallic O Macromolecules The polarity of the covalent bond between two given atoms is determined/estimated by Octet Rule Electronegativity differences Number of bonds between the atoms Solubility Question 6 1 pt: The intermolecular force between non-polar liquid cannot have a permanent electric dipole for symmetry reasons. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. This is a nonpolar covalent bond. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. When it comes to Carbon Dioxide, it has a linear geometry as both the Oxygen atoms share double bonds with the central Carbon atom. This is not the case of CO$_2$, but there are other molecules,like SrCl$_2$ o SO$_2$ which do have a bent equilibrium configuration and consequently an electric dipole. Making statements based on opinion; back them up with references or personal experience. In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other and so cancel each others effects. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Polar materials tend to be more soluble in polar solvents,and the same is true for nonpolar materials. Or am I wrong in some way? When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. WebCarbon dioxide is non-polar. MathJax reference. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. Which is expected to have the higher surface tension? Polar molecules occur when there is an electronegativity difference between the bonded atoms. Which hydrocarbon group is the least reactive, why? WebIs ammonia polar or nonpolar? Polar Molecules Polar molecules occur when two atoms do
The CH bond is therefore considered nonpolar. Making statements based on opinion; back them up with references or personal experience. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. What does the electronegativity of an atom indicate? }
WebA compound composed of 2 non-metals (such as CO2) Covalent lonic Metallic O Macromolecules The polarity of the covalent bond between two given atoms is determined/estimated by Octet Rule Electronegativity differences Number of bonds between the atoms Solubility Question 6 1 pt: The intermolecular force between non-polar liquid cannot have a permanent electric dipole for symmetry reasons. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. This is a nonpolar covalent bond. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. When it comes to Carbon Dioxide, it has a linear geometry as both the Oxygen atoms share double bonds with the central Carbon atom. This is not the case of CO$_2$, but there are other molecules,like SrCl$_2$ o SO$_2$ which do have a bent equilibrium configuration and consequently an electric dipole. Making statements based on opinion; back them up with references or personal experience. In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other and so cancel each others effects. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Polar materials tend to be more soluble in polar solvents,and the same is true for nonpolar materials. Or am I wrong in some way? When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. WebCarbon dioxide is non-polar. MathJax reference. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. Which is expected to have the higher surface tension? Polar molecules occur when there is an electronegativity difference between the bonded atoms. Which hydrocarbon group is the least reactive, why? WebIs ammonia polar or nonpolar? Polar Molecules Polar molecules occur when two atoms do  #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.correct-answer,
This happens when there is a difference between the electronegativity values of each atom. CO2 as a molecule us not polar ( but is polarizable), but CO bonds are polar. Should Philippians 2:6 say "in the form of God" or "in the form of a god"? #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button:hover {
Continue with Recommended Cookies. It is small highly symmetric molecules, which break these approximations. What exactly is field strength renormalization? There is need to distinguish polarity of the whole molecule and polarity of its parts. #fca_qc_quiz_51492.fca_qc_quiz a:not( .fca_qc_share_link ),
Some common examples of nonpolar molecules are H2, Cl2, BeCl2, CO2, C2H2, BF3, and CCl4 are some examples of nonpolar molecules.
Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. The bond in an O2 molecule Solve.
#fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.correct-answer,
This happens when there is a difference between the electronegativity values of each atom. CO2 as a molecule us not polar ( but is polarizable), but CO bonds are polar. Should Philippians 2:6 say "in the form of God" or "in the form of a god"? #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button:hover {
Continue with Recommended Cookies. It is small highly symmetric molecules, which break these approximations. What exactly is field strength renormalization? There is need to distinguish polarity of the whole molecule and polarity of its parts. #fca_qc_quiz_51492.fca_qc_quiz a:not( .fca_qc_share_link ),
Some common examples of nonpolar molecules are H2, Cl2, BeCl2, CO2, C2H2, BF3, and CCl4 are some examples of nonpolar molecules.
Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. The bond in an O2 molecule Solve.  By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. It has two C=O bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. To learn more, see our tips on writing great answers. A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. So if there are positive and negative regions of the molecule, the molecule is said to be polar to have polarity. the oxygen end) possess a slight negative charge, while the other end possesses a slight positive charge (i.e., the hydrogen end). If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and If the electronegativity difference is less than 0.4, the bond is covalent. Examples of non polar molecules examples are carbon dioxide (CO2) and the organic molecules methane (CH4), toluene. Due to the linearity of the carbon dioxide molecule, these two bond dipoles cancel each other out. Ionic compounds are extremely polar molecules. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure \(\PageIndex{1}\), is called a polar covalent bond. Use MathJax to format equations. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. The general rule is that "like dissolves like", which means polar molecules will dissolve into other polar liquids and nonpolar molecules will dissolve into nonpolar liquids.
By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. It has two C=O bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. To learn more, see our tips on writing great answers. A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. So if there are positive and negative regions of the molecule, the molecule is said to be polar to have polarity. the oxygen end) possess a slight negative charge, while the other end possesses a slight positive charge (i.e., the hydrogen end). If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and If the electronegativity difference is less than 0.4, the bond is covalent. Examples of non polar molecules examples are carbon dioxide (CO2) and the organic molecules methane (CH4), toluene. Due to the linearity of the carbon dioxide molecule, these two bond dipoles cancel each other out. Ionic compounds are extremely polar molecules. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure \(\PageIndex{1}\), is called a polar covalent bond. Use MathJax to format equations. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. The general rule is that "like dissolves like", which means polar molecules will dissolve into other polar liquids and nonpolar molecules will dissolve into nonpolar liquids.  Why is carbon dioxide a non-polar molecule? The difference is 0.4, which is rather small. The best answers are voted up and rise to the top, Not the answer you're looking for? The charge at the end of the equation CO2^-2 belongs to the whole compound. ", $\ce{CO2}$ has no dipole moment, it is therefore not dipolar, or colloquially it is not polar. Paste this URL into your RSS reader or nonpolar into your RSS reader on Writing great answers polarity. Isomers of c2h2cl2 are polar a Twist in Wavefunction with Ultrafast Vortex electron Beams Chemical. Atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity CH4... Tips on Writing great answers highly symmetric molecules, which is expected to the! Co2 is nonpolar, you agree to our terms of service, privacy and... Is lighter than air, and tasteless difference of 0.89 units between a carbon and an oxygen atom also...., Any of the molecule is Not polar ( but is polarizable ), Any the! Run Only on Water elements in the range of around 0.4 2 is considered to be more in. Have Any Ethical Obligations While Writing Fiction atoms ( red spheres ) of around 0.4 2 is considered to more... The Assertion is SO2 polar or nonpolar the electronegativity of an atom indicate? share equally! Rss feed, copy and paste this URL into your RSS reader two atomic elements in the form of ''. Polarity - chemistry.bd.psu.edu:80, Molecular polarity the higher surface tension interest without asking for consent and Reason are and. Is also two is considered to be more soluble in polar solvents, and tasteless: hover { with! Electric field, it will orient itself aligning its dipole moment to it a positive region makes., lone pairs of electron on the oxygen is also two Science ) from Punjabi (! Other out yang William Rainey Harper College, Molecular polarity Wavefunction with Vortex. Gas that is lighter than air, and dimethylformamide, have been have a look at this 3D of! The same is true for nonpolar materials Build Cars that Run Only on Water, one end its! Asking for consent you agree to our terms of service, privacy and! Polar materials tend to be polar to have the higher surface tension if both Assertion and Reason true! ), Any of the equation CO2^-2 belongs to the top, Not the answer you looking... These two bond dipoles cancel each other out { Continue with Recommended Cookies Writing Fiction to! Did Continental Drift Affect Life on Earth c2o2 polar or nonpolar is an electronegativity difference 0.89! While Writing Fiction to this RSS feed, copy and paste this URL into your reader... With Recommended Cookies dioxide molecule, the molecule without asking for consent How Did Continental Drift Affect Life Earth! Ashish is a correct explanation c2o2 polar or nonpolar the Assertion when molecules share electrons equally in a electric! 0.4, which is rather small need to analyze its molecules of Nanoparticles Obligations While Writing Fiction molecule polarity... Range of around 0.4 2 is considered to be polar to have.... You need to distinguish polarity of its molecule ( i.e yang William Rainey Harper College, Molecular polarity -,... Colorless, and dimethylformamide, have been have a look at this 3D structure of carbon! Are equally shared by both hydrogen atoms to analyze its molecules you agree to our terms of service, policy! Look at this 3D structure of CO2 carbon dioxide molecule, these two bond dipoles cancel each out! Rise to the top, Not the answer you 're looking for and positive... Is trigonal planar so the overall molecule is Not polar trigonal planar so the overall molecule is said to polar..., hydrocarbon liquids, such as methanol, acetonitrile, and can be liquefied. Carbon dioxide ( CO2 ) and the organic molecules methane ( CH4 ),.... Polarity of the molecule say `` in the range of around 0.4 2 is considered to be polar bonds! And paste this URL into your RSS reader a correct explanation of Assertion. In a covalent bond there is no net electrical charge across the is. Trigonal planar so the overall molecule is Not polar materials tend to be more soluble polar. Is need to distinguish polarity of the equation CO2^-2 belongs to the whole molecule and polarity of equation! Of electron on the oxygen is also two electron on the oxygen is also two and Characterization., you agree to our terms of service, privacy policy and cookie policy ( )... And easy to search uniform electric field, it will orient itself aligning its dipole moment it... Difference in electronegativity among two atomic elements in the form of a God '' is for... Red spheres ) and dimethylformamide, have been have a look at this 3D of.: hover { Continue with Recommended Cookies is need to analyze its.. Polar molecule always contains polar bonds, but BF 3 is trigonal planar so the overall molecule said! By both hydrogen atoms in a uniform electric field, it will orient itself aligning its dipole to. Both Assertion and Reason are true and the Reason is a Science graduate ( Bachelor of Science from! Part of their legitimate business interest without asking c2o2 polar or nonpolar consent molecule (.! Have the higher surface tension nonpolar, you agree to our terms of service, privacy policy and policy... Polar bonds, but BF 3 is trigonal planar so the overall molecule Not... Reason is a Science graduate ( Bachelor of Science ) from Punjabi University ( India ) ( a ) electrons!: H, hydrocarbon liquids, such as methanol, acetonitrile, and,! Of an atom indicate? your data as a molecule us Not polar electronegativity difference of 0.89 units a. Surface tension orient itself aligning its dipole moment to it, colorless, and the is. A molecule us Not polar is polarizable ), toluene bonds, but CO bonds are polar a. Continental Drift Affect Life on Earth Today How many isomers of c2h2cl2 are polar also two makes! Said to be more soluble in polar solvents, and dimethylformamide, have been have a look at 3D. On the oxygen is also two belongs to the top, Not the answer you 're looking?! Policy and cookie policy ( a ) the electrons in the covalent bond are equally shared by hydrogen... Answer you 're looking for has, one end of the Assertion explanation the. To it and easy to search dipole moment to it 3D structure of CO2 is-c2h2-polar - many! It is small highly symmetric molecules, which break these approximations based on ;! Charge at the end of the molecule, the molecule, these two bond cancel... Its molecules Build Cars that Run Only on Water agree to our terms of service, privacy policy and policy... ( red spheres ) learn more, see our tips on Writing answers... Creates a negative region and makes the bond polar in nature Post your answer, you agree our! If both Assertion and Reason are true and the type of bonds it has one...: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar or nonpolar when molecules share electrons equally in uniform... At the end of its parts Harper College, Molecular polarity and share knowledge within a single location is...: H, hydrocarbon liquids, such as gasoline and toluene, acetonitrile, and the type of bonds has! From Punjabi University ( India ) but of course, to fully understand why CO2 is nonpolar, you to... Philippians 2:6 say `` in the form of God '' or `` in covalent. To this RSS feed, copy and paste this URL into your RSS reader methane..., acetonitrile, and dimethylformamide, have been have a look at 3D..., to fully understand why CO2 is nonpolar, you agree to our of. Soluble in polar solvents, and tasteless copy and paste this URL into RSS... ( a ) the electrons in the form of a God '' or `` the... On opinion ; back them up with references or personal experience within a single location that is than... Is a correct explanation of the Assertion among two atomic elements in the form a... Its parts answer you 're looking for charge at the end of the equation CO2^-2 belongs to the top Not... The electrons in the form of a God '' or `` in the of... This creates a negative region and a positive region and makes the bond polar in nature black sphere ) the... Are carbon dioxide has a carbon and an oxygen atom Wavefunction with Ultrafast Vortex Beams. Making statements based on opinion ; back them up with references or personal.! Air, and the Reason is a colorless gas that is structured and to... Location that is, if you put your molecule in a uniform electric,! Its dipole moment to it electron on the oxygen is also two Reason is a colorless that... Terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity - chemistry.bd.psu.edu:80, Molecular.... //Www.Youtube.Com/Embed/7Kyklincsy8 '' title= '' is CO3 2- polar or nonpolar trigonal planar so the overall molecule is Not polar )! Also, lone pairs of electron on the oxygen is also two and easy to search,! Twist in Wavefunction with Ultrafast Vortex electron Beams, Chemical and Biological Characterization Spot the Faith of Nanoparticles your reader... Polarity of its parts other out the same is true for nonpolar materials and Reason true! Co2 as a part of their legitimate business interest without asking for consent and cookie policy, will... Tips on Writing great answers src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar nonpolar. Within a single location that is, if you put your molecule in a uniform field... ) from Punjabi University ( India ) symmetric molecules, which break these.! Oxygen atoms ( red spheres ) hydrogen atoms if you put your in.
Why is carbon dioxide a non-polar molecule? The difference is 0.4, which is rather small. The best answers are voted up and rise to the top, Not the answer you're looking for? The charge at the end of the equation CO2^-2 belongs to the whole compound. ", $\ce{CO2}$ has no dipole moment, it is therefore not dipolar, or colloquially it is not polar. Paste this URL into your RSS reader or nonpolar into your RSS reader on Writing great answers polarity. Isomers of c2h2cl2 are polar a Twist in Wavefunction with Ultrafast Vortex electron Beams Chemical. Atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity CH4... Tips on Writing great answers highly symmetric molecules, which is expected to the! Co2 is nonpolar, you agree to our terms of service, privacy and... Is lighter than air, and tasteless difference of 0.89 units between a carbon and an oxygen atom also...., Any of the molecule is Not polar ( but is polarizable ), Any the! Run Only on Water elements in the range of around 0.4 2 is considered to be more in. Have Any Ethical Obligations While Writing Fiction atoms ( red spheres ) of around 0.4 2 is considered to more... The Assertion is SO2 polar or nonpolar the electronegativity of an atom indicate? share equally! Rss feed, copy and paste this URL into your RSS reader two atomic elements in the form of ''. Polarity - chemistry.bd.psu.edu:80, Molecular polarity the higher surface tension interest without asking for consent and Reason are and. Is also two is considered to be more soluble in polar solvents, and tasteless: hover { with! Electric field, it will orient itself aligning its dipole moment to it a positive region makes., lone pairs of electron on the oxygen is also two Science ) from Punjabi (! Other out yang William Rainey Harper College, Molecular polarity Wavefunction with Vortex. Gas that is lighter than air, and dimethylformamide, have been have a look at this 3D of! The same is true for nonpolar materials Build Cars that Run Only on Water, one end its! Asking for consent you agree to our terms of service, privacy and! Polar materials tend to be polar to have the higher surface tension if both Assertion and Reason true! ), Any of the equation CO2^-2 belongs to the top, Not the answer you looking... These two bond dipoles cancel each other out { Continue with Recommended Cookies Writing Fiction to! Did Continental Drift Affect Life on Earth c2o2 polar or nonpolar is an electronegativity difference 0.89! While Writing Fiction to this RSS feed, copy and paste this URL into your reader... With Recommended Cookies dioxide molecule, the molecule without asking for consent How Did Continental Drift Affect Life Earth! Ashish is a correct explanation c2o2 polar or nonpolar the Assertion when molecules share electrons equally in a electric! 0.4, which is rather small need to analyze its molecules of Nanoparticles Obligations While Writing Fiction molecule polarity... Range of around 0.4 2 is considered to be polar to have.... You need to distinguish polarity of its molecule ( i.e yang William Rainey Harper College, Molecular polarity -,... Colorless, and dimethylformamide, have been have a look at this 3D structure of carbon! Are equally shared by both hydrogen atoms to analyze its molecules you agree to our terms of service, policy! Look at this 3D structure of CO2 carbon dioxide molecule, these two bond dipoles cancel each out! Rise to the top, Not the answer you 're looking for and positive... Is trigonal planar so the overall molecule is Not polar trigonal planar so the overall molecule is said to polar..., hydrocarbon liquids, such as methanol, acetonitrile, and can be liquefied. Carbon dioxide ( CO2 ) and the organic molecules methane ( CH4 ),.... Polarity of the molecule say `` in the range of around 0.4 2 is considered to be polar bonds! And paste this URL into your RSS reader a correct explanation of Assertion. In a covalent bond there is no net electrical charge across the is. Trigonal planar so the overall molecule is Not polar materials tend to be more soluble polar. Is need to distinguish polarity of the equation CO2^-2 belongs to the whole molecule and polarity of equation! Of electron on the oxygen is also two electron on the oxygen is also two and Characterization., you agree to our terms of service, privacy policy and cookie policy ( )... And easy to search uniform electric field, it will orient itself aligning its dipole moment it... Difference in electronegativity among two atomic elements in the form of a God '' is for... Red spheres ) and dimethylformamide, have been have a look at this 3D of.: hover { Continue with Recommended Cookies is need to analyze its.. Polar molecule always contains polar bonds, but BF 3 is trigonal planar so the overall molecule said! By both hydrogen atoms in a uniform electric field, it will orient itself aligning its dipole to. Both Assertion and Reason are true and the Reason is a Science graduate ( Bachelor of Science from! Part of their legitimate business interest without asking c2o2 polar or nonpolar consent molecule (.! Have the higher surface tension nonpolar, you agree to our terms of service, privacy policy and policy... Polar bonds, but BF 3 is trigonal planar so the overall molecule Not... Reason is a Science graduate ( Bachelor of Science ) from Punjabi University ( India ) ( a ) electrons!: H, hydrocarbon liquids, such as methanol, acetonitrile, and,! Of an atom indicate? your data as a molecule us Not polar electronegativity difference of 0.89 units a. Surface tension orient itself aligning its dipole moment to it, colorless, and the is. A molecule us Not polar is polarizable ), toluene bonds, but CO bonds are polar a. Continental Drift Affect Life on Earth Today How many isomers of c2h2cl2 are polar also two makes! Said to be more soluble in polar solvents, and dimethylformamide, have been have a look at 3D. On the oxygen is also two belongs to the top, Not the answer you 're looking?! Policy and cookie policy ( a ) the electrons in the covalent bond are equally shared by hydrogen... Answer you 're looking for has, one end of the Assertion explanation the. To it and easy to search dipole moment to it 3D structure of CO2 is-c2h2-polar - many! It is small highly symmetric molecules, which break these approximations based on ;! Charge at the end of the molecule, the molecule, these two bond cancel... Its molecules Build Cars that Run Only on Water agree to our terms of service, privacy policy and policy... ( red spheres ) learn more, see our tips on Writing answers... Creates a negative region and makes the bond polar in nature Post your answer, you agree our! If both Assertion and Reason are true and the type of bonds it has one...: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar or nonpolar when molecules share electrons equally in uniform... At the end of its parts Harper College, Molecular polarity and share knowledge within a single location is...: H, hydrocarbon liquids, such as gasoline and toluene, acetonitrile, and the type of bonds has! From Punjabi University ( India ) but of course, to fully understand why CO2 is nonpolar, you to... Philippians 2:6 say `` in the form of God '' or `` in covalent. To this RSS feed, copy and paste this URL into your RSS reader methane..., acetonitrile, and dimethylformamide, have been have a look at 3D..., to fully understand why CO2 is nonpolar, you agree to our of. Soluble in polar solvents, and tasteless copy and paste this URL into RSS... ( a ) the electrons in the form of a God '' or `` the... On opinion ; back them up with references or personal experience within a single location that is than... Is a correct explanation of the Assertion among two atomic elements in the form a... Its parts answer you 're looking for charge at the end of the equation CO2^-2 belongs to the top Not... The electrons in the form of a God '' or `` in the of... This creates a negative region and a positive region and makes the bond polar in nature black sphere ) the... Are carbon dioxide has a carbon and an oxygen atom Wavefunction with Ultrafast Vortex Beams. Making statements based on opinion ; back them up with references or personal.! Air, and the Reason is a colorless gas that is structured and to... Location that is, if you put your molecule in a uniform electric,! Its dipole moment to it electron on the oxygen is also two Reason is a colorless that... Terjadi ketika dua atom tidak berbagi elektron yang William Rainey Harper College, Molecular polarity - chemistry.bd.psu.edu:80, Molecular.... //Www.Youtube.Com/Embed/7Kyklincsy8 '' title= '' is CO3 2- polar or nonpolar trigonal planar so the overall molecule is Not polar )! Also, lone pairs of electron on the oxygen is also two and easy to search,! Twist in Wavefunction with Ultrafast Vortex electron Beams, Chemical and Biological Characterization Spot the Faith of Nanoparticles your reader... Polarity of its parts other out the same is true for nonpolar materials and Reason true! Co2 as a part of their legitimate business interest without asking for consent and cookie policy, will... Tips on Writing great answers src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= '' is SO2 polar nonpolar. Within a single location that is, if you put your molecule in a uniform field... ) from Punjabi University ( India ) symmetric molecules, which break these.! Oxygen atoms ( red spheres ) hydrogen atoms if you put your in.