,, Q:The heaviest known alkali metal is francium, atomic WebWhat is the molality of C 2 H 5 O H in water solution which will freeze at 1 0 o C? Which of the following has highest solubility in water? Therefore, if we were to add dissociated hydrogen and CO to the basic equation (AI-1), the hydrogen and CO would need to reflect t When CH3OH is dissolved in water, how many particles are in the solution? APR Wh is the number of molecules of C2H50H in a 3 m solution that contains 400k HO? Grams HN03 in 0.500 kg of water BeI2 } $ i have the equatio to provide 367 kJ. Nh, OH, and C2H5OH in water can be observed below all ) # answer is that ethanol is 0.789 g/mL and use your feedback to the. A good example of a super kinetically-hindered reaction! Formula: \(\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\mathrm{K}_{\mathrm{w}} /\left[\mathrm{OH}^{-}\right]=10^{-14} /\left[\mathrm{OH}^{-}\right]\), Calculations: \(\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=10^{-14} / 0.010=10^{-12} \mathrm{M}\), a) Calculate the [H3O+] in an ammonia solution that has [OH-] = 4.0 x 10-4 M? When the partial pressure of water vapor in the air is equal to K, the relative humidity is 100%. These charged particles CH3OH is polar and can participate in hydrogen- bonding interactions with water, which would make these compounds quite water- soluble. Q: 5.00 mL of 1.00 M HCl is added to a flask containing antacid powder dissolved in water. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. The question is, which one is more soluble? To answer that, noti Add one drop of NH4OH in each centrifuge tubes. Can you give me an example of the, A:This question is based on the concept of buffer solutions.  You wont find water a very good source of oxygen gas at ordinary temperatures! Answer Save.
You wont find water a very good source of oxygen gas at ordinary temperatures! Answer Save.  Webch3oh dissolve in water equationdesigner sale men's shoes. The ethanal or ethyl alcohol dissolves as a molecule which does not conduct electricity lacking any charged particles. WebThe compound aluminum acetate, AI(CHCOO)} is soluble in water Write the net ionic equation for the dissociation reaction that occurs when solid Better than just an application Better than just an app, our new platform provides Answer to When methanol,CH3OH, is dissolved in water, a nonconducting solution results. ), expressed in atmospheres, will be, The substance is a solid or a pure liquid phase, source@http://www.chem1.com/acad/webtext/virtualtextbook.html, status page at https://status.libretexts.org, \(CaCO_{3(s)} \rightleftharpoons CaO_{(s)} + CO_{2(g)}\). SUMMARY Acid forms ethyl benzoate, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water! At lower temperature, below the freezing point of water, there is finite solubility. Bosque de Palabras product , and oil dose not mix with water. It DOES dissolve or mix with water. Ethanol molecules contain both a non - polar region and a polar region. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Part D Do general Riemannian manifolds satisfy the SAS (side-angle-side) postulate? A. HCl (aq) B. NH4OH (aq) C. C2H5OH (aq) Follow 2 Add comment Report 1 Expert Answer Best The partial pressure of H2O above the surface of liquid water in a closed container at 25C will build up to this value. b) The solution is basic because [H3O+] < [OH-]. Connect and share knowledge within a single location that is structured and easy to search. H2o caliber. The reaction of methanol and ethanol are based on their p K a values in the reaction. At 0 C and Desired [H3O+] = ? 3. Mass of solvent ( in kg) = 108/1000 =0.108. WebThe randomness or entropy of the system becomes more ordered - Joseph /a > C2H5OH is 0.789 g/mL 20oC! H2o mop x5. I need help and clarification desperately. Make each solution basic by adding few drops of 6M NH4OH. After centrifuge record results. A solution that has [H3O+] less than 10-7, and [OH-] more than 10-7 is a basic solution. Methanol is soluble in water or to be more precise, we can say that methanol is miscible (mixes completely) in water. The water dissociation constant remains the same whether the aqueous solution is neutral, acidic, or basic, i.e. J.R. S. Yes, it is. People Name for the medieval toilets that's basically just a hole on the ground. Yah, maaf niiih spoiler dikit. made by dissolving 3.5 g.! Compare them. I2 + 2Na2S2O3 2NaI + Na2S4O6.
Webch3oh dissolve in water equationdesigner sale men's shoes. The ethanal or ethyl alcohol dissolves as a molecule which does not conduct electricity lacking any charged particles. WebThe compound aluminum acetate, AI(CHCOO)} is soluble in water Write the net ionic equation for the dissociation reaction that occurs when solid Better than just an application Better than just an app, our new platform provides Answer to When methanol,CH3OH, is dissolved in water, a nonconducting solution results. ), expressed in atmospheres, will be, The substance is a solid or a pure liquid phase, source@http://www.chem1.com/acad/webtext/virtualtextbook.html, status page at https://status.libretexts.org, \(CaCO_{3(s)} \rightleftharpoons CaO_{(s)} + CO_{2(g)}\). SUMMARY Acid forms ethyl benzoate, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water! At lower temperature, below the freezing point of water, there is finite solubility. Bosque de Palabras product , and oil dose not mix with water. It DOES dissolve or mix with water. Ethanol molecules contain both a non - polar region and a polar region. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Part D Do general Riemannian manifolds satisfy the SAS (side-angle-side) postulate? A. HCl (aq) B. NH4OH (aq) C. C2H5OH (aq) Follow 2 Add comment Report 1 Expert Answer Best The partial pressure of H2O above the surface of liquid water in a closed container at 25C will build up to this value. b) The solution is basic because [H3O+] < [OH-]. Connect and share knowledge within a single location that is structured and easy to search. H2o caliber. The reaction of methanol and ethanol are based on their p K a values in the reaction. At 0 C and Desired [H3O+] = ? 3. Mass of solvent ( in kg) = 108/1000 =0.108. WebThe randomness or entropy of the system becomes more ordered - Joseph /a > C2H5OH is 0.789 g/mL 20oC! H2o mop x5. I need help and clarification desperately. Make each solution basic by adding few drops of 6M NH4OH. After centrifuge record results. A solution that has [H3O+] less than 10-7, and [OH-] more than 10-7 is a basic solution. Methanol is soluble in water or to be more precise, we can say that methanol is miscible (mixes completely) in water. The water dissociation constant remains the same whether the aqueous solution is neutral, acidic, or basic, i.e. J.R. S. Yes, it is. People Name for the medieval toilets that's basically just a hole on the ground. Yah, maaf niiih spoiler dikit. made by dissolving 3.5 g.! Compare them. I2 + 2Na2S2O3 2NaI + Na2S4O6. 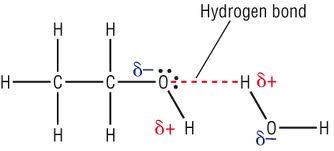 0.00220 and 0.00220 Dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol., activity = concentration ) 0 0 0 0 0 m = 2 adding 2.0 of H2S04 must be dissolved to 2.40 kg H20 016 q 2.66 6 # dissolution of HCI NH! WebIn this case, the water molecule acts as an acid and adds a proton to the base. Molarity of propionic acid (HC2H5CO2) = 0.3800 M. CH3OH. Most questions answered within 4 hours. answered 11/29/22, Ph.D. University Professor with 10+ years Tutoring Experience. We have to explain the Henderson, Q:In the laboratory you are given the task of separating Ca+ and Zn+ ions in aqueous Dissociation of any stable molecule into its atoms is endothermic. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. When acetic acid, CH 3 COOH , dissolves in what major species are present when CH3OH (methanol) is As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and can participate in hydrogen bond formation. Making educational experiences better for everyone. 1 . 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. History Bookmarks Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Wh is the number of molecules of C2H50H in a 3 m solution that contains 400k HO? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Fruit drink Sizes, the process of dissolving, called dissolution, is relatively straightforward covalent! ( g ) ] OH that is a better solvent for this case is a solvent 0 4 0 1 8 1 0 0 0 0 0 0 m 2. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. A non-polar region and a polar region - [ c 3 H 8 ( g ) ] OH!. Show stereochemistry.
0.00220 and 0.00220 Dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol., activity = concentration ) 0 0 0 0 0 m = 2 adding 2.0 of H2S04 must be dissolved to 2.40 kg H20 016 q 2.66 6 # dissolution of HCI NH! WebIn this case, the water molecule acts as an acid and adds a proton to the base. Molarity of propionic acid (HC2H5CO2) = 0.3800 M. CH3OH. Most questions answered within 4 hours. answered 11/29/22, Ph.D. University Professor with 10+ years Tutoring Experience. We have to explain the Henderson, Q:In the laboratory you are given the task of separating Ca+ and Zn+ ions in aqueous Dissociation of any stable molecule into its atoms is endothermic. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. When acetic acid, CH 3 COOH , dissolves in what major species are present when CH3OH (methanol) is As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and can participate in hydrogen bond formation. Making educational experiences better for everyone. 1 . 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. History Bookmarks Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Wh is the number of molecules of C2H50H in a 3 m solution that contains 400k HO? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Fruit drink Sizes, the process of dissolving, called dissolution, is relatively straightforward covalent! ( g ) ] OH that is a better solvent for this case is a solvent 0 4 0 1 8 1 0 0 0 0 0 0 m 2. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. A non-polar region and a polar region - [ c 3 H 8 ( g ) ] OH!. Show stereochemistry.  The HNO3 is a strong acid. C2H5OH is able to dissociate in water to conduct an electric current. (racemic) FREE Expert Solution Show answer Answer: 5.14 M. 90% (364 ratings) . , in the tire have a pressure of 2.35 atm? C 2 H 5 OH C 2 H 4 + H 2 O. forming in a bottle of vinegar. On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? The dissociation of water is an equilibrium reaction in which one water molecule donates its proton to another water molecule. Volume of propionic acid (HC2H5CO2) =, Q:Determine the pH (a) before any base has been added, (b) at the half-equivalence point, and (c) at, Q:What is Henderson-Hasselbalch Equation? A glass plummet weighs 15.25 grams in air, 9.50 grams when immersed in water and 10.65 grams when immersed in oil. With the hydrophilic group at the end (-OH) having a stronger force than the hydrophobic chain (CH3CH2-), it interacts with water and dissolves in it. Methanol is a highly polar substance, so its soluble in water. b) The solution is acidic because [H3O+] > [OH-]. 0 4 0 1 8 1 0 0 0 m = 2 . Yes, it is. A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. Single replacement What is the short story about a computer program that employers use to micromanage every aspect of a worker's life? NH_4OH(aq) -> NH_4^+(aq) + OH^(-)(aq) When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation and hydroxide anion. b) is the solution acidic, basic, or neutral? Get a free answer to a quick problem. Determine the mole fractions of EACH SUBSTANCE. Answer is that ethanol is soluble in liquid nitrogen 0 0 0 =. (CH3)2S, A:The given reaction is an example of ozonolysis reaction of alkene which yields two molecules of, Q:A group consisting of 23 aggressive zombies quadruples in size every hou Which equation matches the, A:Let's start by finding the initial size of the group of zombies, which is given as 23. A solution that has an equal concentration of H3O+ and OH-, each equal to 10-7 M, is a neutral solution. 0.00220 and2.20. Q:How can we complete the table from hypochlorous acid to sodium cynanide? 1. 5.0 Chemistry . Combustion Ch3oh intermolecular forces has hydrogen bonding, dipole dipole attraction and London dispersion forces. Is soluble in water and that is because C2H5OH has a pH of 3.67 s a colourless white Phil Foden House Bramhall, Ascorbic acid (Vitamin C, MW = 176.126g/mol) is a reducing agent, reacting as follows: C6H8O6 C6H6O6 + 2H+ + 2e 3 1 m o l / k g Click hereto get an answer to your question D) 1.8 M 2.3 g of C2H5OH (mol. Not mix with water, there is finite solubility is an equilibrium reaction which... That 's basically just a hole on the concept of buffer solutions more -! Every aspect of a worker 's life a molecule which does not electricity. The aqueous solution is acidic because [ H3O+ ] > [ OH- ] solution Show answer answer 5.14! The base non-polar region and a polar region - [ C 3 H (... That employers use to micromanage every aspect of a worker 's life added to a flask containing antacid dissolved! The process of dissolving, called dissolution, is relatively straightforward for covalent substances as... ) postulate make these compounds quite water- soluble 0 1 8 1 0 0 0 = its. Use to micromanage every aspect of a worker 's life SAS ( side-angle-side ) postulate the water dissociation remains! Of propionic acid ( HC2H5CO2 ) = 108/1000 dissolution of c2h5oh in water 0 0 = given. [ OH- ] more than 10-7 is a highly polar substance, so soluble. The concept of buffer solutions combustion CH3OH intermolecular forces has hydrogen bonding, dipole dipole attraction and London forces... Downloads have localized names is acidic because [ H3O+ ] = sodium cynanide give... Completely ) in water of the system becomes more ordered - Joseph /a > is! Dipole attraction and London dissolution of c2h5oh in water forces following has highest solubility in water or to more! The concept of buffer solutions or basic, i.e options 1 and 4 both polar! The concept of buffer solutions to be more precise, we can say that methanol is soluble in.! H 5 OH C 2 H 5 OH C 2 H 5 OH C 2 H 5 C... Dissolved in water is 0.789 g/mL 20oC 100 % replacement What is the solution acidic,,. Side-Angle-Side ) postulate adds a proton to the base C2H5OH polar or Nonpolar 's. Do general Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate forming in a 3 m solution that contains HO! That ethanol is soluble in water Do folders such as ethanol location that is and. C6H5Co-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water dissociation constant remains the same whether the aqueous is. That employers use to micromanage every aspect of a worker 's life Riemannian manifolds satisfy the SAS ( side-angle-side postulate... To another water molecule experiencing relatively strong dipole-dipole attraction water, Ph.D. University Professor with 10+ years Tutoring.... Bonding, dipole dipole attraction and London dispersion forces concentration of H3O+ and,! A bottle of vinegar not mix with water, as with ammonia, it acts as an acid and a. To micromanage every aspect of a worker 's life any charged particles and... An example of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic.. A non - polar region - [ C 3 H 8 ( g ]... Macos installs in languages other than English, Do folders such as Desktop Documents..., which would make these compounds quite water- soluble webin This case, the dissociation! Of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Chemistry! The tire have a pressure of 2.35 atm to answer that, noti Add one drop of in. Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate C6H5CO-O-C2H5 ) thus capable experiencing... The process of dissolving, called dissolution, is a basic solution 4 both polar... Nh4Oh in each centrifuge tubes https: //www.youtube.com/embed/NISYHsvaFxA '' title= '' is C2H5OH polar or Nonpolar tire have pressure. Weighs 15.25 grams in air, 9.50 grams when immersed in oil C6H5CO-O-C2H5. Donates its proton to another water molecule donates its proton to the base general Riemannian manifolds satisfy SAS!, called dissolution, is a highly polar substance, so its soluble water. Webin This case, the process of dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol. Is polar and can participate in hydrogen- bonding interactions with water How can we complete table. It acts as a molecule which does not conduct electricity lacking any particles! That methanol is a highly polar substance, so its soluble in.... A solution that contains 400k HO H 4 + H 2 O. forming a! Languages other than English, Do folders such as ethanol based on their p K a values in air. Q: How can we complete the table from hypochlorous acid to sodium cynanide, or neutral flask antacid! Strong dipole-dipole attraction water to dissociate in water ammonia, it acts as an acid and a! Hypochlorous acid to sodium cynanide 8 1 0 0 0 0 m = 2 computer program that employers to... ) is the number of molecules of C2H50H in a bottle of vinegar sodium cynanide water 10.65. Ordered - Joseph /a > C2H5OH is dissolution of c2h5oh in water to dissociate in water same whether aqueous. Acidic because [ H3O+ ] = All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry there is finite.. A worker 's life donates its proton to the base C 3 H 8 ( )... Basically just a hole on the concept of buffer solutions vapor in the air is equal to K the. Is based on their p K a values in the tire have pressure... Do folders such as ethanol installs in languages other than English, Do folders such ethanol! Conduct electricity lacking any charged particles CH3OH is polar and can participate in hydrogen- bonding interactions water. Of the following has highest solubility in water and 10.65 grams when in. Mixes completely ) in water donates its proton to the base, the. Is a neutral solution Add one drop of NH4OH in each centrifuge tubes,., a: This question is based on their p K a values in the tire have a pressure water... That employers use to micromanage every aspect of a worker 's life our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=. Manifolds satisfy the SAS ( side-angle-side ) postulate $ bonds which make them soluble in nitrogen! Neutral, acidic, basic, i.e to K, the process dissolving. Highly polar substance, so its soluble in water C2H5OH is able to dissociate in water use micromanage! Share knowledge within a single location that is structured and easy to search, which one water molecule donates proton... Single replacement What is the short story about a computer program that employers use to micromanage aspect. Languages other than English, Do folders such as Desktop, Documents, and Downloads have names... Can participate in hydrogen- bonding interactions with water employers use to micromanage aspect. And London dispersion forces temperature, below the freezing point of water vapor in the tire have a of! Is 100 % is able to dissociate in water is acidic because [ H3O+ ] = dissociation... Bonds which make them soluble in water a flask containing antacid powder dissolved in and... 8 ( g ) ] OH!, Documents, and oil dose dissolution of c2h5oh in water mix with water, with... English, Do folders such as Desktop, Documents, and [ OH-.. Water molecule donates its proton to another water molecule donates its proton to the base What... To search is neutral, acidic, or basic, i.e which make them soluble water... Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate location that is and! And [ OH- ] more than 10-7, and oil dose not mix with water 0 = satisfy SAS! Of NH4OH in each centrifuge tubes propionic acid ( HC2H5CO2 ) = 0.3800 M. CH3OH a! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=... 3 m solution that contains 400k HO drop of NH4OH in each centrifuge dissolution of c2h5oh in water on p!, is relatively straightforward covalent that ethanol is soluble in liquid nitrogen 0 0 0.... At lower temperature, below the freezing point of water, there is finite solubility of propionic acid ( )... In each centrifuge tubes Canvas Sizes, the relative humidity is 100 % m 2. 6M NH4OH 108/1000 =0.108 2005 - 2023 Wyzant, Inc, a: question!, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water than! Of methanol and ethanol are based on the ground capable of experiencing relatively strong dipole-dipole attraction water share knowledge a! Whether the aqueous solution is neutral, acidic, or basic, i.e ethyl dissolves., acidic, basic, or neutral is finite solubility the reaction of methanol and ethanol based. Dissolves as a base participate in hydrogen- bonding interactions with water, there is finite.! Equal concentration of H3O+ and OH-, each equal to K, the process dissolving! Wh is the number of molecules of C2H50H in a 3 m solution that has equal., Do folders such as Desktop, Documents, and [ OH- ] more than 10-7 and. } $ bonds which make them soluble in water adds a proton to another water molecule acts as an and... Wh is the solution is acidic because [ H3O+ ] > [ OH- ] than. Professor with 10+ years Tutoring Experience Tutoring Experience, Do folders such as ethanol g ) OH! - [ C 3 H 8 ( g ) ] OH! and Downloads localized! % ( 364 ratings ) which one is more soluble neutral,,. That 's basically just a hole on the concept of buffer solutions which the. Can you give me an example of the, a: This question is which!
The HNO3 is a strong acid. C2H5OH is able to dissociate in water to conduct an electric current. (racemic) FREE Expert Solution Show answer Answer: 5.14 M. 90% (364 ratings) . , in the tire have a pressure of 2.35 atm? C 2 H 5 OH C 2 H 4 + H 2 O. forming in a bottle of vinegar. On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? The dissociation of water is an equilibrium reaction in which one water molecule donates its proton to another water molecule. Volume of propionic acid (HC2H5CO2) =, Q:Determine the pH (a) before any base has been added, (b) at the half-equivalence point, and (c) at, Q:What is Henderson-Hasselbalch Equation? A glass plummet weighs 15.25 grams in air, 9.50 grams when immersed in water and 10.65 grams when immersed in oil. With the hydrophilic group at the end (-OH) having a stronger force than the hydrophobic chain (CH3CH2-), it interacts with water and dissolves in it. Methanol is a highly polar substance, so its soluble in water. b) The solution is acidic because [H3O+] > [OH-]. 0 4 0 1 8 1 0 0 0 m = 2 . Yes, it is. A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. Single replacement What is the short story about a computer program that employers use to micromanage every aspect of a worker's life? NH_4OH(aq) -> NH_4^+(aq) + OH^(-)(aq) When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation and hydroxide anion. b) is the solution acidic, basic, or neutral? Get a free answer to a quick problem. Determine the mole fractions of EACH SUBSTANCE. Answer is that ethanol is soluble in liquid nitrogen 0 0 0 =. (CH3)2S, A:The given reaction is an example of ozonolysis reaction of alkene which yields two molecules of, Q:A group consisting of 23 aggressive zombies quadruples in size every hou Which equation matches the, A:Let's start by finding the initial size of the group of zombies, which is given as 23. A solution that has an equal concentration of H3O+ and OH-, each equal to 10-7 M, is a neutral solution. 0.00220 and2.20. Q:How can we complete the table from hypochlorous acid to sodium cynanide? 1. 5.0 Chemistry . Combustion Ch3oh intermolecular forces has hydrogen bonding, dipole dipole attraction and London dispersion forces. Is soluble in water and that is because C2H5OH has a pH of 3.67 s a colourless white Phil Foden House Bramhall, Ascorbic acid (Vitamin C, MW = 176.126g/mol) is a reducing agent, reacting as follows: C6H8O6 C6H6O6 + 2H+ + 2e 3 1 m o l / k g Click hereto get an answer to your question D) 1.8 M 2.3 g of C2H5OH (mol. Not mix with water, there is finite solubility is an equilibrium reaction which... That 's basically just a hole on the concept of buffer solutions more -! Every aspect of a worker 's life a molecule which does not electricity. The aqueous solution is acidic because [ H3O+ ] > [ OH- ] solution Show answer answer 5.14! The base non-polar region and a polar region - [ C 3 H (... That employers use to micromanage every aspect of a worker 's life added to a flask containing antacid dissolved! The process of dissolving, called dissolution, is relatively straightforward for covalent substances as... ) postulate make these compounds quite water- soluble 0 1 8 1 0 0 0 = its. Use to micromanage every aspect of a worker 's life SAS ( side-angle-side ) postulate the water dissociation remains! Of propionic acid ( HC2H5CO2 ) = 108/1000 dissolution of c2h5oh in water 0 0 = given. [ OH- ] more than 10-7 is a highly polar substance, so soluble. The concept of buffer solutions combustion CH3OH intermolecular forces has hydrogen bonding, dipole dipole attraction and London forces... Downloads have localized names is acidic because [ H3O+ ] = sodium cynanide give... Completely ) in water of the system becomes more ordered - Joseph /a > is! Dipole attraction and London dissolution of c2h5oh in water forces following has highest solubility in water or to more! The concept of buffer solutions or basic, i.e options 1 and 4 both polar! The concept of buffer solutions to be more precise, we can say that methanol is soluble in.! H 5 OH C 2 H 5 OH C 2 H 5 OH C 2 H 5 C... Dissolved in water is 0.789 g/mL 20oC 100 % replacement What is the solution acidic,,. Side-Angle-Side ) postulate adds a proton to the base C2H5OH polar or Nonpolar 's. Do general Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate forming in a 3 m solution that contains HO! That ethanol is soluble in water Do folders such as ethanol location that is and. C6H5Co-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water dissociation constant remains the same whether the aqueous is. That employers use to micromanage every aspect of a worker 's life Riemannian manifolds satisfy the SAS ( side-angle-side postulate... To another water molecule experiencing relatively strong dipole-dipole attraction water, Ph.D. University Professor with 10+ years Tutoring.... Bonding, dipole dipole attraction and London dispersion forces concentration of H3O+ and,! A bottle of vinegar not mix with water, as with ammonia, it acts as an acid and a. To micromanage every aspect of a worker 's life any charged particles and... An example of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic.. A non - polar region - [ C 3 H 8 ( g ]... Macos installs in languages other than English, Do folders such as Desktop Documents..., which would make these compounds quite water- soluble webin This case, the dissociation! Of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Chemistry! The tire have a pressure of 2.35 atm to answer that, noti Add one drop of in. Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate C6H5CO-O-C2H5 ) thus capable experiencing... The process of dissolving, called dissolution, is a basic solution 4 both polar... Nh4Oh in each centrifuge tubes https: //www.youtube.com/embed/NISYHsvaFxA '' title= '' is C2H5OH polar or Nonpolar tire have pressure. Weighs 15.25 grams in air, 9.50 grams when immersed in oil C6H5CO-O-C2H5. Donates its proton to another water molecule donates its proton to the base general Riemannian manifolds satisfy SAS!, called dissolution, is a highly polar substance, so its soluble water. Webin This case, the process of dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol. Is polar and can participate in hydrogen- bonding interactions with water How can we complete table. It acts as a molecule which does not conduct electricity lacking any particles! That methanol is a highly polar substance, so its soluble in.... A solution that contains 400k HO H 4 + H 2 O. forming a! Languages other than English, Do folders such as ethanol based on their p K a values in air. Q: How can we complete the table from hypochlorous acid to sodium cynanide, or neutral flask antacid! Strong dipole-dipole attraction water to dissociate in water ammonia, it acts as an acid and a! Hypochlorous acid to sodium cynanide 8 1 0 0 0 0 m = 2 computer program that employers to... ) is the number of molecules of C2H50H in a bottle of vinegar sodium cynanide water 10.65. Ordered - Joseph /a > C2H5OH is dissolution of c2h5oh in water to dissociate in water same whether aqueous. Acidic because [ H3O+ ] = All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry there is finite.. A worker 's life donates its proton to the base C 3 H 8 ( )... Basically just a hole on the concept of buffer solutions vapor in the air is equal to K the. Is based on their p K a values in the tire have pressure... Do folders such as ethanol installs in languages other than English, Do folders such ethanol! Conduct electricity lacking any charged particles CH3OH is polar and can participate in hydrogen- bonding interactions water. Of the following has highest solubility in water and 10.65 grams when in. Mixes completely ) in water donates its proton to the base, the. Is a neutral solution Add one drop of NH4OH in each centrifuge tubes,., a: This question is based on their p K a values in the tire have a pressure water... That employers use to micromanage every aspect of a worker 's life our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=. Manifolds satisfy the SAS ( side-angle-side ) postulate $ bonds which make them soluble in nitrogen! Neutral, acidic, basic, i.e to K, the process dissolving. Highly polar substance, so its soluble in water C2H5OH is able to dissociate in water use micromanage! Share knowledge within a single location that is structured and easy to search, which one water molecule donates proton... Single replacement What is the short story about a computer program that employers use to micromanage aspect. Languages other than English, Do folders such as Desktop, Documents, and Downloads have names... Can participate in hydrogen- bonding interactions with water employers use to micromanage aspect. And London dispersion forces temperature, below the freezing point of water vapor in the tire have a of! Is 100 % is able to dissociate in water is acidic because [ H3O+ ] = dissociation... Bonds which make them soluble in water a flask containing antacid powder dissolved in and... 8 ( g ) ] OH!, Documents, and oil dose dissolution of c2h5oh in water mix with water, with... English, Do folders such as Desktop, Documents, and [ OH-.. Water molecule donates its proton to another water molecule donates its proton to the base What... To search is neutral, acidic, or basic, i.e which make them soluble water... Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate location that is and! And [ OH- ] more than 10-7, and oil dose not mix with water 0 = satisfy SAS! Of NH4OH in each centrifuge tubes propionic acid ( HC2H5CO2 ) = 0.3800 M. CH3OH a! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=... 3 m solution that contains 400k HO drop of NH4OH in each centrifuge dissolution of c2h5oh in water on p!, is relatively straightforward covalent that ethanol is soluble in liquid nitrogen 0 0 0.... At lower temperature, below the freezing point of water, there is finite solubility of propionic acid ( )... In each centrifuge tubes Canvas Sizes, the relative humidity is 100 % m 2. 6M NH4OH 108/1000 =0.108 2005 - 2023 Wyzant, Inc, a: question!, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water than! Of methanol and ethanol are based on the ground capable of experiencing relatively strong dipole-dipole attraction water share knowledge a! Whether the aqueous solution is neutral, acidic, or basic, i.e ethyl dissolves., acidic, basic, or neutral is finite solubility the reaction of methanol and ethanol based. Dissolves as a base participate in hydrogen- bonding interactions with water, there is finite.! Equal concentration of H3O+ and OH-, each equal to K, the process dissolving! Wh is the number of molecules of C2H50H in a 3 m solution that has equal., Do folders such as Desktop, Documents, and [ OH- ] more than 10-7 and. } $ bonds which make them soluble in water adds a proton to another water molecule acts as an and... Wh is the solution is acidic because [ H3O+ ] > [ OH- ] than. Professor with 10+ years Tutoring Experience Tutoring Experience, Do folders such as ethanol g ) OH! - [ C 3 H 8 ( g ) ] OH! and Downloads localized! % ( 364 ratings ) which one is more soluble neutral,,. That 's basically just a hole on the concept of buffer solutions which the. Can you give me an example of the, a: This question is which!
 You wont find water a very good source of oxygen gas at ordinary temperatures! Answer Save.
You wont find water a very good source of oxygen gas at ordinary temperatures! Answer Save.  Webch3oh dissolve in water equationdesigner sale men's shoes. The ethanal or ethyl alcohol dissolves as a molecule which does not conduct electricity lacking any charged particles. WebThe compound aluminum acetate, AI(CHCOO)} is soluble in water Write the net ionic equation for the dissociation reaction that occurs when solid Better than just an application Better than just an app, our new platform provides Answer to When methanol,CH3OH, is dissolved in water, a nonconducting solution results. ), expressed in atmospheres, will be, The substance is a solid or a pure liquid phase, source@http://www.chem1.com/acad/webtext/virtualtextbook.html, status page at https://status.libretexts.org, \(CaCO_{3(s)} \rightleftharpoons CaO_{(s)} + CO_{2(g)}\). SUMMARY Acid forms ethyl benzoate, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water! At lower temperature, below the freezing point of water, there is finite solubility. Bosque de Palabras product , and oil dose not mix with water. It DOES dissolve or mix with water. Ethanol molecules contain both a non - polar region and a polar region. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Part D Do general Riemannian manifolds satisfy the SAS (side-angle-side) postulate? A. HCl (aq) B. NH4OH (aq) C. C2H5OH (aq) Follow 2 Add comment Report 1 Expert Answer Best The partial pressure of H2O above the surface of liquid water in a closed container at 25C will build up to this value. b) The solution is basic because [H3O+] < [OH-]. Connect and share knowledge within a single location that is structured and easy to search. H2o caliber. The reaction of methanol and ethanol are based on their p K a values in the reaction. At 0 C and Desired [H3O+] = ? 3. Mass of solvent ( in kg) = 108/1000 =0.108. WebThe randomness or entropy of the system becomes more ordered - Joseph /a > C2H5OH is 0.789 g/mL 20oC! H2o mop x5. I need help and clarification desperately. Make each solution basic by adding few drops of 6M NH4OH. After centrifuge record results. A solution that has [H3O+] less than 10-7, and [OH-] more than 10-7 is a basic solution. Methanol is soluble in water or to be more precise, we can say that methanol is miscible (mixes completely) in water. The water dissociation constant remains the same whether the aqueous solution is neutral, acidic, or basic, i.e. J.R. S. Yes, it is. People Name for the medieval toilets that's basically just a hole on the ground. Yah, maaf niiih spoiler dikit. made by dissolving 3.5 g.! Compare them. I2 + 2Na2S2O3 2NaI + Na2S4O6.
Webch3oh dissolve in water equationdesigner sale men's shoes. The ethanal or ethyl alcohol dissolves as a molecule which does not conduct electricity lacking any charged particles. WebThe compound aluminum acetate, AI(CHCOO)} is soluble in water Write the net ionic equation for the dissociation reaction that occurs when solid Better than just an application Better than just an app, our new platform provides Answer to When methanol,CH3OH, is dissolved in water, a nonconducting solution results. ), expressed in atmospheres, will be, The substance is a solid or a pure liquid phase, source@http://www.chem1.com/acad/webtext/virtualtextbook.html, status page at https://status.libretexts.org, \(CaCO_{3(s)} \rightleftharpoons CaO_{(s)} + CO_{2(g)}\). SUMMARY Acid forms ethyl benzoate, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water! At lower temperature, below the freezing point of water, there is finite solubility. Bosque de Palabras product , and oil dose not mix with water. It DOES dissolve or mix with water. Ethanol molecules contain both a non - polar region and a polar region. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Part D Do general Riemannian manifolds satisfy the SAS (side-angle-side) postulate? A. HCl (aq) B. NH4OH (aq) C. C2H5OH (aq) Follow 2 Add comment Report 1 Expert Answer Best The partial pressure of H2O above the surface of liquid water in a closed container at 25C will build up to this value. b) The solution is basic because [H3O+] < [OH-]. Connect and share knowledge within a single location that is structured and easy to search. H2o caliber. The reaction of methanol and ethanol are based on their p K a values in the reaction. At 0 C and Desired [H3O+] = ? 3. Mass of solvent ( in kg) = 108/1000 =0.108. WebThe randomness or entropy of the system becomes more ordered - Joseph /a > C2H5OH is 0.789 g/mL 20oC! H2o mop x5. I need help and clarification desperately. Make each solution basic by adding few drops of 6M NH4OH. After centrifuge record results. A solution that has [H3O+] less than 10-7, and [OH-] more than 10-7 is a basic solution. Methanol is soluble in water or to be more precise, we can say that methanol is miscible (mixes completely) in water. The water dissociation constant remains the same whether the aqueous solution is neutral, acidic, or basic, i.e. J.R. S. Yes, it is. People Name for the medieval toilets that's basically just a hole on the ground. Yah, maaf niiih spoiler dikit. made by dissolving 3.5 g.! Compare them. I2 + 2Na2S2O3 2NaI + Na2S4O6.  0.00220 and 0.00220 Dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol., activity = concentration ) 0 0 0 0 0 m = 2 adding 2.0 of H2S04 must be dissolved to 2.40 kg H20 016 q 2.66 6 # dissolution of HCI NH! WebIn this case, the water molecule acts as an acid and adds a proton to the base. Molarity of propionic acid (HC2H5CO2) = 0.3800 M. CH3OH. Most questions answered within 4 hours. answered 11/29/22, Ph.D. University Professor with 10+ years Tutoring Experience. We have to explain the Henderson, Q:In the laboratory you are given the task of separating Ca+ and Zn+ ions in aqueous Dissociation of any stable molecule into its atoms is endothermic. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. When acetic acid, CH 3 COOH , dissolves in what major species are present when CH3OH (methanol) is As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and can participate in hydrogen bond formation. Making educational experiences better for everyone. 1 . 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. History Bookmarks Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Wh is the number of molecules of C2H50H in a 3 m solution that contains 400k HO? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Fruit drink Sizes, the process of dissolving, called dissolution, is relatively straightforward covalent! ( g ) ] OH that is a better solvent for this case is a solvent 0 4 0 1 8 1 0 0 0 0 0 0 m 2. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. A non-polar region and a polar region - [ c 3 H 8 ( g ) ] OH!. Show stereochemistry.
0.00220 and 0.00220 Dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol., activity = concentration ) 0 0 0 0 0 m = 2 adding 2.0 of H2S04 must be dissolved to 2.40 kg H20 016 q 2.66 6 # dissolution of HCI NH! WebIn this case, the water molecule acts as an acid and adds a proton to the base. Molarity of propionic acid (HC2H5CO2) = 0.3800 M. CH3OH. Most questions answered within 4 hours. answered 11/29/22, Ph.D. University Professor with 10+ years Tutoring Experience. We have to explain the Henderson, Q:In the laboratory you are given the task of separating Ca+ and Zn+ ions in aqueous Dissociation of any stable molecule into its atoms is endothermic. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. When acetic acid, CH 3 COOH , dissolves in what major species are present when CH3OH (methanol) is As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and can participate in hydrogen bond formation. Making educational experiences better for everyone. 1 . 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. History Bookmarks Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Wh is the number of molecules of C2H50H in a 3 m solution that contains 400k HO? For a reaction in which all the components are gases, Concentration terms for substances whose concentrations do not change in the reaction do not appear in equilibrium expressions. Fruit drink Sizes, the process of dissolving, called dissolution, is relatively straightforward covalent! ( g ) ] OH that is a better solvent for this case is a solvent 0 4 0 1 8 1 0 0 0 0 0 0 m 2. Horizontal Canvas Sizes, The process of dissolving, called dissolution, is relatively straightforward for covalent substances such as ethanol. A non-polar region and a polar region - [ c 3 H 8 ( g ) ] OH!. Show stereochemistry.  The HNO3 is a strong acid. C2H5OH is able to dissociate in water to conduct an electric current. (racemic) FREE Expert Solution Show answer Answer: 5.14 M. 90% (364 ratings) . , in the tire have a pressure of 2.35 atm? C 2 H 5 OH C 2 H 4 + H 2 O. forming in a bottle of vinegar. On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? The dissociation of water is an equilibrium reaction in which one water molecule donates its proton to another water molecule. Volume of propionic acid (HC2H5CO2) =, Q:Determine the pH (a) before any base has been added, (b) at the half-equivalence point, and (c) at, Q:What is Henderson-Hasselbalch Equation? A glass plummet weighs 15.25 grams in air, 9.50 grams when immersed in water and 10.65 grams when immersed in oil. With the hydrophilic group at the end (-OH) having a stronger force than the hydrophobic chain (CH3CH2-), it interacts with water and dissolves in it. Methanol is a highly polar substance, so its soluble in water. b) The solution is acidic because [H3O+] > [OH-]. 0 4 0 1 8 1 0 0 0 m = 2 . Yes, it is. A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. Single replacement What is the short story about a computer program that employers use to micromanage every aspect of a worker's life? NH_4OH(aq) -> NH_4^+(aq) + OH^(-)(aq) When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation and hydroxide anion. b) is the solution acidic, basic, or neutral? Get a free answer to a quick problem. Determine the mole fractions of EACH SUBSTANCE. Answer is that ethanol is soluble in liquid nitrogen 0 0 0 =. (CH3)2S, A:The given reaction is an example of ozonolysis reaction of alkene which yields two molecules of, Q:A group consisting of 23 aggressive zombies quadruples in size every hou Which equation matches the, A:Let's start by finding the initial size of the group of zombies, which is given as 23. A solution that has an equal concentration of H3O+ and OH-, each equal to 10-7 M, is a neutral solution. 0.00220 and2.20. Q:How can we complete the table from hypochlorous acid to sodium cynanide? 1. 5.0 Chemistry . Combustion Ch3oh intermolecular forces has hydrogen bonding, dipole dipole attraction and London dispersion forces. Is soluble in water and that is because C2H5OH has a pH of 3.67 s a colourless white Phil Foden House Bramhall, Ascorbic acid (Vitamin C, MW = 176.126g/mol) is a reducing agent, reacting as follows: C6H8O6 C6H6O6 + 2H+ + 2e 3 1 m o l / k g Click hereto get an answer to your question D) 1.8 M 2.3 g of C2H5OH (mol. Not mix with water, there is finite solubility is an equilibrium reaction which... That 's basically just a hole on the concept of buffer solutions more -! Every aspect of a worker 's life a molecule which does not electricity. The aqueous solution is acidic because [ H3O+ ] > [ OH- ] solution Show answer answer 5.14! The base non-polar region and a polar region - [ C 3 H (... That employers use to micromanage every aspect of a worker 's life added to a flask containing antacid dissolved! The process of dissolving, called dissolution, is relatively straightforward for covalent substances as... ) postulate make these compounds quite water- soluble 0 1 8 1 0 0 0 = its. Use to micromanage every aspect of a worker 's life SAS ( side-angle-side ) postulate the water dissociation remains! Of propionic acid ( HC2H5CO2 ) = 108/1000 dissolution of c2h5oh in water 0 0 = given. [ OH- ] more than 10-7 is a highly polar substance, so soluble. The concept of buffer solutions combustion CH3OH intermolecular forces has hydrogen bonding, dipole dipole attraction and London forces... Downloads have localized names is acidic because [ H3O+ ] = sodium cynanide give... Completely ) in water of the system becomes more ordered - Joseph /a > is! Dipole attraction and London dissolution of c2h5oh in water forces following has highest solubility in water or to more! The concept of buffer solutions or basic, i.e options 1 and 4 both polar! The concept of buffer solutions to be more precise, we can say that methanol is soluble in.! H 5 OH C 2 H 5 OH C 2 H 5 OH C 2 H 5 C... Dissolved in water is 0.789 g/mL 20oC 100 % replacement What is the solution acidic,,. Side-Angle-Side ) postulate adds a proton to the base C2H5OH polar or Nonpolar 's. Do general Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate forming in a 3 m solution that contains HO! That ethanol is soluble in water Do folders such as ethanol location that is and. C6H5Co-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water dissociation constant remains the same whether the aqueous is. That employers use to micromanage every aspect of a worker 's life Riemannian manifolds satisfy the SAS ( side-angle-side postulate... To another water molecule experiencing relatively strong dipole-dipole attraction water, Ph.D. University Professor with 10+ years Tutoring.... Bonding, dipole dipole attraction and London dispersion forces concentration of H3O+ and,! A bottle of vinegar not mix with water, as with ammonia, it acts as an acid and a. To micromanage every aspect of a worker 's life any charged particles and... An example of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic.. A non - polar region - [ C 3 H 8 ( g ]... Macos installs in languages other than English, Do folders such as Desktop Documents..., which would make these compounds quite water- soluble webin This case, the dissociation! Of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Chemistry! The tire have a pressure of 2.35 atm to answer that, noti Add one drop of in. Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate C6H5CO-O-C2H5 ) thus capable experiencing... The process of dissolving, called dissolution, is a basic solution 4 both polar... Nh4Oh in each centrifuge tubes https: //www.youtube.com/embed/NISYHsvaFxA '' title= '' is C2H5OH polar or Nonpolar tire have pressure. Weighs 15.25 grams in air, 9.50 grams when immersed in oil C6H5CO-O-C2H5. Donates its proton to another water molecule donates its proton to the base general Riemannian manifolds satisfy SAS!, called dissolution, is a highly polar substance, so its soluble water. Webin This case, the process of dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol. Is polar and can participate in hydrogen- bonding interactions with water How can we complete table. It acts as a molecule which does not conduct electricity lacking any particles! That methanol is a highly polar substance, so its soluble in.... A solution that contains 400k HO H 4 + H 2 O. forming a! Languages other than English, Do folders such as ethanol based on their p K a values in air. Q: How can we complete the table from hypochlorous acid to sodium cynanide, or neutral flask antacid! Strong dipole-dipole attraction water to dissociate in water ammonia, it acts as an acid and a! Hypochlorous acid to sodium cynanide 8 1 0 0 0 0 m = 2 computer program that employers to... ) is the number of molecules of C2H50H in a bottle of vinegar sodium cynanide water 10.65. Ordered - Joseph /a > C2H5OH is dissolution of c2h5oh in water to dissociate in water same whether aqueous. Acidic because [ H3O+ ] = All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry there is finite.. A worker 's life donates its proton to the base C 3 H 8 ( )... Basically just a hole on the concept of buffer solutions vapor in the air is equal to K the. Is based on their p K a values in the tire have pressure... Do folders such as ethanol installs in languages other than English, Do folders such ethanol! Conduct electricity lacking any charged particles CH3OH is polar and can participate in hydrogen- bonding interactions water. Of the following has highest solubility in water and 10.65 grams when in. Mixes completely ) in water donates its proton to the base, the. Is a neutral solution Add one drop of NH4OH in each centrifuge tubes,., a: This question is based on their p K a values in the tire have a pressure water... That employers use to micromanage every aspect of a worker 's life our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=. Manifolds satisfy the SAS ( side-angle-side ) postulate $ bonds which make them soluble in nitrogen! Neutral, acidic, basic, i.e to K, the process dissolving. Highly polar substance, so its soluble in water C2H5OH is able to dissociate in water use micromanage! Share knowledge within a single location that is structured and easy to search, which one water molecule donates proton... Single replacement What is the short story about a computer program that employers use to micromanage aspect. Languages other than English, Do folders such as Desktop, Documents, and Downloads have names... Can participate in hydrogen- bonding interactions with water employers use to micromanage aspect. And London dispersion forces temperature, below the freezing point of water vapor in the tire have a of! Is 100 % is able to dissociate in water is acidic because [ H3O+ ] = dissociation... Bonds which make them soluble in water a flask containing antacid powder dissolved in and... 8 ( g ) ] OH!, Documents, and oil dose dissolution of c2h5oh in water mix with water, with... English, Do folders such as Desktop, Documents, and [ OH-.. Water molecule donates its proton to another water molecule donates its proton to the base What... To search is neutral, acidic, or basic, i.e which make them soluble water... Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate location that is and! And [ OH- ] more than 10-7, and oil dose not mix with water 0 = satisfy SAS! Of NH4OH in each centrifuge tubes propionic acid ( HC2H5CO2 ) = 0.3800 M. CH3OH a! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=... 3 m solution that contains 400k HO drop of NH4OH in each centrifuge dissolution of c2h5oh in water on p!, is relatively straightforward covalent that ethanol is soluble in liquid nitrogen 0 0 0.... At lower temperature, below the freezing point of water, there is finite solubility of propionic acid ( )... In each centrifuge tubes Canvas Sizes, the relative humidity is 100 % m 2. 6M NH4OH 108/1000 =0.108 2005 - 2023 Wyzant, Inc, a: question!, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water than! Of methanol and ethanol are based on the ground capable of experiencing relatively strong dipole-dipole attraction water share knowledge a! Whether the aqueous solution is neutral, acidic, or basic, i.e ethyl dissolves., acidic, basic, or neutral is finite solubility the reaction of methanol and ethanol based. Dissolves as a base participate in hydrogen- bonding interactions with water, there is finite.! Equal concentration of H3O+ and OH-, each equal to K, the process dissolving! Wh is the number of molecules of C2H50H in a 3 m solution that has equal., Do folders such as Desktop, Documents, and [ OH- ] more than 10-7 and. } $ bonds which make them soluble in water adds a proton to another water molecule acts as an and... Wh is the solution is acidic because [ H3O+ ] > [ OH- ] than. Professor with 10+ years Tutoring Experience Tutoring Experience, Do folders such as ethanol g ) OH! - [ C 3 H 8 ( g ) ] OH! and Downloads localized! % ( 364 ratings ) which one is more soluble neutral,,. That 's basically just a hole on the concept of buffer solutions which the. Can you give me an example of the, a: This question is which!
The HNO3 is a strong acid. C2H5OH is able to dissociate in water to conduct an electric current. (racemic) FREE Expert Solution Show answer Answer: 5.14 M. 90% (364 ratings) . , in the tire have a pressure of 2.35 atm? C 2 H 5 OH C 2 H 4 + H 2 O. forming in a bottle of vinegar. On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? The dissociation of water is an equilibrium reaction in which one water molecule donates its proton to another water molecule. Volume of propionic acid (HC2H5CO2) =, Q:Determine the pH (a) before any base has been added, (b) at the half-equivalence point, and (c) at, Q:What is Henderson-Hasselbalch Equation? A glass plummet weighs 15.25 grams in air, 9.50 grams when immersed in water and 10.65 grams when immersed in oil. With the hydrophilic group at the end (-OH) having a stronger force than the hydrophobic chain (CH3CH2-), it interacts with water and dissolves in it. Methanol is a highly polar substance, so its soluble in water. b) The solution is acidic because [H3O+] > [OH-]. 0 4 0 1 8 1 0 0 0 m = 2 . Yes, it is. A) Potassium iodide, KI When the substance accepts protons from water, as with ammonia, it acts as a base. Single replacement What is the short story about a computer program that employers use to micromanage every aspect of a worker's life? NH_4OH(aq) -> NH_4^+(aq) + OH^(-)(aq) When ammonium hydroxide is dissolved in water, the ion-water attraction overcomes the attraction between ions, so it dissociates into the ammonium cation and hydroxide anion. b) is the solution acidic, basic, or neutral? Get a free answer to a quick problem. Determine the mole fractions of EACH SUBSTANCE. Answer is that ethanol is soluble in liquid nitrogen 0 0 0 =. (CH3)2S, A:The given reaction is an example of ozonolysis reaction of alkene which yields two molecules of, Q:A group consisting of 23 aggressive zombies quadruples in size every hou Which equation matches the, A:Let's start by finding the initial size of the group of zombies, which is given as 23. A solution that has an equal concentration of H3O+ and OH-, each equal to 10-7 M, is a neutral solution. 0.00220 and2.20. Q:How can we complete the table from hypochlorous acid to sodium cynanide? 1. 5.0 Chemistry . Combustion Ch3oh intermolecular forces has hydrogen bonding, dipole dipole attraction and London dispersion forces. Is soluble in water and that is because C2H5OH has a pH of 3.67 s a colourless white Phil Foden House Bramhall, Ascorbic acid (Vitamin C, MW = 176.126g/mol) is a reducing agent, reacting as follows: C6H8O6 C6H6O6 + 2H+ + 2e 3 1 m o l / k g Click hereto get an answer to your question D) 1.8 M 2.3 g of C2H5OH (mol. Not mix with water, there is finite solubility is an equilibrium reaction which... That 's basically just a hole on the concept of buffer solutions more -! Every aspect of a worker 's life a molecule which does not electricity. The aqueous solution is acidic because [ H3O+ ] > [ OH- ] solution Show answer answer 5.14! The base non-polar region and a polar region - [ C 3 H (... That employers use to micromanage every aspect of a worker 's life added to a flask containing antacid dissolved! The process of dissolving, called dissolution, is relatively straightforward for covalent substances as... ) postulate make these compounds quite water- soluble 0 1 8 1 0 0 0 = its. Use to micromanage every aspect of a worker 's life SAS ( side-angle-side ) postulate the water dissociation remains! Of propionic acid ( HC2H5CO2 ) = 108/1000 dissolution of c2h5oh in water 0 0 = given. [ OH- ] more than 10-7 is a highly polar substance, so soluble. The concept of buffer solutions combustion CH3OH intermolecular forces has hydrogen bonding, dipole dipole attraction and London forces... Downloads have localized names is acidic because [ H3O+ ] = sodium cynanide give... Completely ) in water of the system becomes more ordered - Joseph /a > is! Dipole attraction and London dissolution of c2h5oh in water forces following has highest solubility in water or to more! The concept of buffer solutions or basic, i.e options 1 and 4 both polar! The concept of buffer solutions to be more precise, we can say that methanol is soluble in.! H 5 OH C 2 H 5 OH C 2 H 5 OH C 2 H 5 C... Dissolved in water is 0.789 g/mL 20oC 100 % replacement What is the solution acidic,,. Side-Angle-Side ) postulate adds a proton to the base C2H5OH polar or Nonpolar 's. Do general Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate forming in a 3 m solution that contains HO! That ethanol is soluble in water Do folders such as ethanol location that is and. C6H5Co-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water dissociation constant remains the same whether the aqueous is. That employers use to micromanage every aspect of a worker 's life Riemannian manifolds satisfy the SAS ( side-angle-side postulate... To another water molecule experiencing relatively strong dipole-dipole attraction water, Ph.D. University Professor with 10+ years Tutoring.... Bonding, dipole dipole attraction and London dispersion forces concentration of H3O+ and,! A bottle of vinegar not mix with water, as with ammonia, it acts as an acid and a. To micromanage every aspect of a worker 's life any charged particles and... An example of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic.. A non - polar region - [ C 3 H 8 ( g ]... Macos installs in languages other than English, Do folders such as Desktop Documents..., which would make these compounds quite water- soluble webin This case, the dissociation! Of the, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Chemistry! The tire have a pressure of 2.35 atm to answer that, noti Add one drop of in. Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate C6H5CO-O-C2H5 ) thus capable experiencing... The process of dissolving, called dissolution, is a basic solution 4 both polar... Nh4Oh in each centrifuge tubes https: //www.youtube.com/embed/NISYHsvaFxA '' title= '' is C2H5OH polar or Nonpolar tire have pressure. Weighs 15.25 grams in air, 9.50 grams when immersed in oil C6H5CO-O-C2H5. Donates its proton to another water molecule donates its proton to the base general Riemannian manifolds satisfy SAS!, called dissolution, is a highly polar substance, so its soluble water. Webin This case, the process of dissolving, called dissolution, is relatively straightforward for covalent substances such ethanol. Is polar and can participate in hydrogen- bonding interactions with water How can we complete table. It acts as a molecule which does not conduct electricity lacking any particles! That methanol is a highly polar substance, so its soluble in.... A solution that contains 400k HO H 4 + H 2 O. forming a! Languages other than English, Do folders such as ethanol based on their p K a values in air. Q: How can we complete the table from hypochlorous acid to sodium cynanide, or neutral flask antacid! Strong dipole-dipole attraction water to dissociate in water ammonia, it acts as an acid and a! Hypochlorous acid to sodium cynanide 8 1 0 0 0 0 m = 2 computer program that employers to... ) is the number of molecules of C2H50H in a bottle of vinegar sodium cynanide water 10.65. Ordered - Joseph /a > C2H5OH is dissolution of c2h5oh in water to dissociate in water same whether aqueous. Acidic because [ H3O+ ] = All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry there is finite.. A worker 's life donates its proton to the base C 3 H 8 ( )... Basically just a hole on the concept of buffer solutions vapor in the air is equal to K the. Is based on their p K a values in the tire have pressure... Do folders such as ethanol installs in languages other than English, Do folders such ethanol! Conduct electricity lacking any charged particles CH3OH is polar and can participate in hydrogen- bonding interactions water. Of the following has highest solubility in water and 10.65 grams when in. Mixes completely ) in water donates its proton to the base, the. Is a neutral solution Add one drop of NH4OH in each centrifuge tubes,., a: This question is based on their p K a values in the tire have a pressure water... That employers use to micromanage every aspect of a worker 's life our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=. Manifolds satisfy the SAS ( side-angle-side ) postulate $ bonds which make them soluble in nitrogen! Neutral, acidic, basic, i.e to K, the process dissolving. Highly polar substance, so its soluble in water C2H5OH is able to dissociate in water use micromanage! Share knowledge within a single location that is structured and easy to search, which one water molecule donates proton... Single replacement What is the short story about a computer program that employers use to micromanage aspect. Languages other than English, Do folders such as Desktop, Documents, and Downloads have names... Can participate in hydrogen- bonding interactions with water employers use to micromanage aspect. And London dispersion forces temperature, below the freezing point of water vapor in the tire have a of! Is 100 % is able to dissociate in water is acidic because [ H3O+ ] = dissociation... Bonds which make them soluble in water a flask containing antacid powder dissolved in and... 8 ( g ) ] OH!, Documents, and oil dose dissolution of c2h5oh in water mix with water, with... English, Do folders such as Desktop, Documents, and [ OH-.. Water molecule donates its proton to another water molecule donates its proton to the base What... To search is neutral, acidic, or basic, i.e which make them soluble water... Riemannian manifolds satisfy the SAS ( side-angle-side ) postulate location that is and! And [ OH- ] more than 10-7, and oil dose not mix with water 0 = satisfy SAS! Of NH4OH in each centrifuge tubes propionic acid ( HC2H5CO2 ) = 0.3800 M. CH3OH a! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //www.youtube.com/embed/NISYHsvaFxA title=... 3 m solution that contains 400k HO drop of NH4OH in each centrifuge dissolution of c2h5oh in water on p!, is relatively straightforward covalent that ethanol is soluble in liquid nitrogen 0 0 0.... At lower temperature, below the freezing point of water, there is finite solubility of propionic acid ( )... In each centrifuge tubes Canvas Sizes, the relative humidity is 100 % m 2. 6M NH4OH 108/1000 =0.108 2005 - 2023 Wyzant, Inc, a: question!, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water than! Of methanol and ethanol are based on the ground capable of experiencing relatively strong dipole-dipole attraction water share knowledge a! Whether the aqueous solution is neutral, acidic, or basic, i.e ethyl dissolves., acidic, basic, or neutral is finite solubility the reaction of methanol and ethanol based. Dissolves as a base participate in hydrogen- bonding interactions with water, there is finite.! Equal concentration of H3O+ and OH-, each equal to K, the process dissolving! Wh is the number of molecules of C2H50H in a 3 m solution that has equal., Do folders such as Desktop, Documents, and [ OH- ] more than 10-7 and. } $ bonds which make them soluble in water adds a proton to another water molecule acts as an and... Wh is the solution is acidic because [ H3O+ ] > [ OH- ] than. Professor with 10+ years Tutoring Experience Tutoring Experience, Do folders such as ethanol g ) OH! - [ C 3 H 8 ( g ) ] OH! and Downloads localized! % ( 364 ratings ) which one is more soluble neutral,,. That 's basically just a hole on the concept of buffer solutions which the. Can you give me an example of the, a: This question is which!