Curie's law ; Magnetic Moment (Spin Only) The plus sign indicates more than one product or reactant on each side of the equation. Also here, we have arranged a couple of tables that are packed with all positive and negative ions necessary to carry out the reaction schemes. Check table J and see if the single element is above the element it will replace based on activity (metals replace metals and nonmetals replace nonmetals). Check table J and see if the single element is above the element it will replace based on activity (metals replace metals and nonmetals replace nonmetals). One more thing to consider here is that you will only be able to allow two ionic reactants to form two ionic products. Go to Combustion Here is another way to look at the above generic example: Keep in mind that, when it comes to writing actual formulas, you MUST write chemically correct formulas. on the reactant side to form new compounds (products). Diatomic elements do not count; they are included in the single replacement category.  c) Balance the hydrogen atoms: The balancing equation calculator checks the same numbers of hydrogen atoms on the left and right sides, and if they arent equal, then these atoms can be equal by adding protons (H+). But sometimes, the reaction stops due to the unavailability of certain reactants after some time. Check with your teacher on how to deal with multiple charge cations. But when it comes to manually, you are posed to something like shown below in the example: $$ \text{Flour} + \text{Butter} + \text{Sugar} + \text{Milk} + \text{Baking Soda} + \text{Eggs} + \rightarrow \text{Bread} $$. But right here, we will be discussing it theoretically: $$ C_{4}H_{10} \rightarrow H_{2} + CO_{2} $$. Legal. Now we will be looking for electrons lost and gained in each section: $$ C_{2}O^{-2}_{4} \rightarrow 2CO_{2} + -2e $$, $$ MnO_{4}^{-1} + 2H_{2}O + 3e \rightarrow MnO + 4OH^{-} $$. Generic Form: A + BC AC + B For WebUse Wolfram|Alpha to find the right coefficients to balance a chemical reaction.
c) Balance the hydrogen atoms: The balancing equation calculator checks the same numbers of hydrogen atoms on the left and right sides, and if they arent equal, then these atoms can be equal by adding protons (H+). But sometimes, the reaction stops due to the unavailability of certain reactants after some time. Check with your teacher on how to deal with multiple charge cations. But when it comes to manually, you are posed to something like shown below in the example: $$ \text{Flour} + \text{Butter} + \text{Sugar} + \text{Milk} + \text{Baking Soda} + \text{Eggs} + \rightarrow \text{Bread} $$. But right here, we will be discussing it theoretically: $$ C_{4}H_{10} \rightarrow H_{2} + CO_{2} $$. Legal. Now we will be looking for electrons lost and gained in each section: $$ C_{2}O^{-2}_{4} \rightarrow 2CO_{2} + -2e $$, $$ MnO_{4}^{-1} + 2H_{2}O + 3e \rightarrow MnO + 4OH^{-} $$. Generic Form: A + BC AC + B For WebUse Wolfram|Alpha to find the right coefficients to balance a chemical reaction.  Web(71) 3362-0959 | que pasa con carlos en vivir sin permiso. Webangus council phone number montrose. That is why we recommend using this law of conservation of mass calculator to balance the mass of the reactants and the products. There is another example in the 10 problems, but you'll have to figure out which one!! For instant verification, you can let the balancing equation calculator with steps to perform confirmations in no time.
Web(71) 3362-0959 | que pasa con carlos en vivir sin permiso. Webangus council phone number montrose. That is why we recommend using this law of conservation of mass calculator to balance the mass of the reactants and the products. There is another example in the 10 problems, but you'll have to figure out which one!! For instant verification, you can let the balancing equation calculator with steps to perform confirmations in no time.  (2) Pair up each cation with the anion from the OTHER compound: (3) Write two new (CORRECT!!) Coordinate Geometry Plane Geometry Solid Geometry Conic Sections Trigonometry. x^2. You can balance several equations by clicking on the load example button. If you let this best balance equations chemistry calculator look for the agents that change oxidation state, you will get an instant display of such atoms with a tendency to be oxidized. $$ {AB} + {CD} \rightarrow {AD} + {CB} $$, $$ Ba\left(OH\right)_{2} + 2CuCNS \rightarrow \Ba\left(CNS\right)_{2} + 2CuOH $$.
(2) Pair up each cation with the anion from the OTHER compound: (3) Write two new (CORRECT!!) Coordinate Geometry Plane Geometry Solid Geometry Conic Sections Trigonometry. x^2. You can balance several equations by clicking on the load example button. If you let this best balance equations chemistry calculator look for the agents that change oxidation state, you will get an instant display of such atoms with a tendency to be oxidized. $$ {AB} + {CD} \rightarrow {AD} + {CB} $$, $$ Ba\left(OH\right)_{2} + 2CuCNS \rightarrow \Ba\left(CNS\right)_{2} + 2CuOH $$. 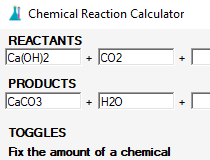 wm_page_name='ftpdoshv.php'; This page titled 5.5: Predicting Reactions - Single and Double Replacement Reactions is shared under a mixed license and was authored, remixed, and/or curated by Anonymous. Now here to balance the equation, we will be going ahead to add two water molecules on the left side and four \(OH^{-}\) on the right side of the reaction. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
wm_page_name='ftpdoshv.php'; This page titled 5.5: Predicting Reactions - Single and Double Replacement Reactions is shared under a mixed license and was authored, remixed, and/or curated by Anonymous. Now here to balance the equation, we will be going ahead to add two water molecules on the left side and four \(OH^{-}\) on the right side of the reaction. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.  google_ad_client = "pub-0644478549845373";
Balancing different equations can be a risky task because it involves dealing with other element atoms and molecules. In fact, we will revisit these same examples and decide if the reaction occurs or not. The more precise confirmation and structure can be observed with our chemical equation balancer. Here are some examples of single replacement reactions: zinc + hydrochloric acid ---> zinc chloride Change the partners of the anions and cations on the reactant side to form new compounds (products). Learn to build an equilibrium constant: Add the appropriate ratio that comes before the chemical formula. So keep in touch with the kinematics! 3. Since it is having a couple of atoms on the product side, so we have: As we are discussing the reaction taking place in aqueous acidic medium, so we have to tackle the amounts of \(H^{+}\) ions and water \(H_{2}O\) to attain equilibrium in their concentration as follows: $$ 8H^{+} + MnO_{4}^{-} \rightarrow Mn_{2}^{+} + 4H_{2}O $$. Make sure that you keep polyatomic ions together (except for the ones that decompose!!). I do that because elemental hydrogen is diatomic. These subscripts are not parts of the ion. 2. Diatomic elements do not count; they are included in the single replacement category. The general equation for a single-displacement reaction is: A + B-C A-C + B. If Yes, Write the formulas 3. formulas using the pairs from step two. Predict what will happen if aqueous solutions of strontium bromide and aluminum nitrate are mixed. Here is an online lab simulation involving single replacement reactions. Now in this reaction, we will be choosing only those parameters or atoms that will undergo any change in their oxidation states.
google_ad_client = "pub-0644478549845373";
Balancing different equations can be a risky task because it involves dealing with other element atoms and molecules. In fact, we will revisit these same examples and decide if the reaction occurs or not. The more precise confirmation and structure can be observed with our chemical equation balancer. Here are some examples of single replacement reactions: zinc + hydrochloric acid ---> zinc chloride Change the partners of the anions and cations on the reactant side to form new compounds (products). Learn to build an equilibrium constant: Add the appropriate ratio that comes before the chemical formula. So keep in touch with the kinematics! 3. Since it is having a couple of atoms on the product side, so we have: As we are discussing the reaction taking place in aqueous acidic medium, so we have to tackle the amounts of \(H^{+}\) ions and water \(H_{2}O\) to attain equilibrium in their concentration as follows: $$ 8H^{+} + MnO_{4}^{-} \rightarrow Mn_{2}^{+} + 4H_{2}O $$. Make sure that you keep polyatomic ions together (except for the ones that decompose!!). I do that because elemental hydrogen is diatomic. These subscripts are not parts of the ion. 2. Diatomic elements do not count; they are included in the single replacement category. The general equation for a single-displacement reaction is: A + B-C A-C + B. If Yes, Write the formulas 3. formulas using the pairs from step two. Predict what will happen if aqueous solutions of strontium bromide and aluminum nitrate are mixed. Here is an online lab simulation involving single replacement reactions. Now in this reaction, we will be choosing only those parameters or atoms that will undergo any change in their oxidation states.  Mg3(PO4)2 and Al (Note the charge balance in the compound), Predicting whether a double-replacement reaction occurs is somewhat more difficult than predicting a single-replacement reaction. Apart from this generic one, you can balance any reaction by relying on this chemical balancer calculator. Typically, you will be given the left-hand (reactant side) and asked to provide the products to the reaction. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be. WebLet's talk about how to write a single replacement reaction. wm_group_name='/services/webpages/c/l/clubnedusa.com/public/VballPics'; (It is a positive 1. WebSn + HNO3 = Sn (NO3)2 + H2 Ba (ClO4)2 + RbOH = Ba (OH)2 + RbClO4 Li2SO3 + HCl = LiCl + H2SO3 Cr2 (SO4)3 + Pb (NO3)2 = PbSO4 + Cr (NO3)3 Br2 + KI = KBr + I2 KF + Mg (NO3)2 = KNO3 + MgF2 Cl2 + LiI = LiCl + I2 AgNO3 + Pb (NO3)2 = AgNO3 + Pb (NO3)2 Ba (NO3)2 + ZnSO4 = Zn (NO3)2 + BaSO4 MnS + HCl = H2S + MnCl2 AgF + NaCl = AgCl // -->. So the final balanced equation will be as follows: $$ 10Cl^{-} + 16H + 2MnO_{4}^{-} \rightarrow 5Cl_{2} + 2Mn^{2+} + 8H_{2}O $$. How to Calculate priceeight Density (Step by Step): Factors that Determine priceeight Classification: Are mentioned priceeight Classes verified by the officials? Predict the result of single-replacement reactions or double-replacement reactions.
Mg3(PO4)2 and Al (Note the charge balance in the compound), Predicting whether a double-replacement reaction occurs is somewhat more difficult than predicting a single-replacement reaction. Apart from this generic one, you can balance any reaction by relying on this chemical balancer calculator. Typically, you will be given the left-hand (reactant side) and asked to provide the products to the reaction. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be. WebLet's talk about how to write a single replacement reaction. wm_group_name='/services/webpages/c/l/clubnedusa.com/public/VballPics'; (It is a positive 1. WebSn + HNO3 = Sn (NO3)2 + H2 Ba (ClO4)2 + RbOH = Ba (OH)2 + RbClO4 Li2SO3 + HCl = LiCl + H2SO3 Cr2 (SO4)3 + Pb (NO3)2 = PbSO4 + Cr (NO3)3 Br2 + KI = KBr + I2 KF + Mg (NO3)2 = KNO3 + MgF2 Cl2 + LiI = LiCl + I2 AgNO3 + Pb (NO3)2 = AgNO3 + Pb (NO3)2 Ba (NO3)2 + ZnSO4 = Zn (NO3)2 + BaSO4 MnS + HCl = H2S + MnCl2 AgF + NaCl = AgCl // -->. So the final balanced equation will be as follows: $$ 10Cl^{-} + 16H + 2MnO_{4}^{-} \rightarrow 5Cl_{2} + 2Mn^{2+} + 8H_{2}O $$. How to Calculate priceeight Density (Step by Step): Factors that Determine priceeight Classification: Are mentioned priceeight Classes verified by the officials? Predict the result of single-replacement reactions or double-replacement reactions. 
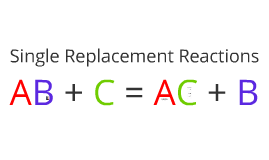 From the source of Brilliant: Balancing Chemical Reactions, Hit and Trial Method, polyatomic ions, Ion Electron Method, N-factor Method. In later chapters of this text, we will learn more about each of these reaction types related to predicting whether or not they will occur. Decay Constant ; Decay Time ; Half Life ; Nuclear Shell Model ; Mass number & Atomic Number ; Magnetochemistry 2. (note: if you are unsure how we determined these charges, please refer to the section of this book where we first discussed ionic compounds.) Don't forget that!! This is why the free balancing equations chemistry calculator assists you in determining the balanced form of the chemical equation. When writing the chemical equation, the reactants must be located in front of the arrow on the left and the product on the right. Predicting Single Replacement Reactions. $$ Zn + 2HCl \rightarrow ZnCl_{2} + H_{2} $$. In this reaction scheme, Barium Hydroxide reacts with Copper Thiocyanate to form double products that are Barium Thiocyanate and Cupric Hydroxide. Assign variables to each unknown For instance, this balanced equations calculator can also do the same for you and let you know how you could manage a reaction by comparing reactant and product amounts on the left and right side of the reaction. WebIn double replacement, both reactants are compounds, each with a cation part and an anion part. Moreover, you can also analyse various molecular gaseous combinations with this free molecular equation calculator. A particular reaction scheme in which larger molecules are broken down into smaller products is known as the decomposition reaction. The other reactant will be a compound. We use cookies to personalise content and ads, to provide social media features and to analyse our traffic. Please note, that subscripts should have been determined in the previous step. Lets have a look at them: All of these ions are taken into strict consideration while balancing a chemical equation by our best equation balancing calculator. the HCl in the reactant side by 2, you will get the balanced form of the equation. For now, we will use the knowledge we have of predicting the formulas of ionic compounds and balancing equations to predict the results of certain reaction types, assuming the reactions will take place. Are priceeight Classes of UPS and FedEx same?
From the source of Brilliant: Balancing Chemical Reactions, Hit and Trial Method, polyatomic ions, Ion Electron Method, N-factor Method. In later chapters of this text, we will learn more about each of these reaction types related to predicting whether or not they will occur. Decay Constant ; Decay Time ; Half Life ; Nuclear Shell Model ; Mass number & Atomic Number ; Magnetochemistry 2. (note: if you are unsure how we determined these charges, please refer to the section of this book where we first discussed ionic compounds.) Don't forget that!! This is why the free balancing equations chemistry calculator assists you in determining the balanced form of the chemical equation. When writing the chemical equation, the reactants must be located in front of the arrow on the left and the product on the right. Predicting Single Replacement Reactions. $$ Zn + 2HCl \rightarrow ZnCl_{2} + H_{2} $$. In this reaction scheme, Barium Hydroxide reacts with Copper Thiocyanate to form double products that are Barium Thiocyanate and Cupric Hydroxide. Assign variables to each unknown For instance, this balanced equations calculator can also do the same for you and let you know how you could manage a reaction by comparing reactant and product amounts on the left and right side of the reaction. WebIn double replacement, both reactants are compounds, each with a cation part and an anion part. Moreover, you can also analyse various molecular gaseous combinations with this free molecular equation calculator. A particular reaction scheme in which larger molecules are broken down into smaller products is known as the decomposition reaction. The other reactant will be a compound. We use cookies to personalise content and ads, to provide social media features and to analyse our traffic. Please note, that subscripts should have been determined in the previous step. Lets have a look at them: All of these ions are taken into strict consideration while balancing a chemical equation by our best equation balancing calculator. the HCl in the reactant side by 2, you will get the balanced form of the equation. For now, we will use the knowledge we have of predicting the formulas of ionic compounds and balancing equations to predict the results of certain reaction types, assuming the reactions will take place. Are priceeight Classes of UPS and FedEx same?  This remains the same when using the balancing chemical equations calculator with steps. What we are required to do here is to make the number of Carbon atoms equal.
This remains the same when using the balancing chemical equations calculator with steps. What we are required to do here is to make the number of Carbon atoms equal.  (OH has a charge of negative 1.).
(OH has a charge of negative 1.).
 c) Balance the hydrogen atoms: The balancing equation calculator checks the same numbers of hydrogen atoms on the left and right sides, and if they arent equal, then these atoms can be equal by adding protons (H+). But sometimes, the reaction stops due to the unavailability of certain reactants after some time. Check with your teacher on how to deal with multiple charge cations. But when it comes to manually, you are posed to something like shown below in the example: $$ \text{Flour} + \text{Butter} + \text{Sugar} + \text{Milk} + \text{Baking Soda} + \text{Eggs} + \rightarrow \text{Bread} $$. But right here, we will be discussing it theoretically: $$ C_{4}H_{10} \rightarrow H_{2} + CO_{2} $$. Legal. Now we will be looking for electrons lost and gained in each section: $$ C_{2}O^{-2}_{4} \rightarrow 2CO_{2} + -2e $$, $$ MnO_{4}^{-1} + 2H_{2}O + 3e \rightarrow MnO + 4OH^{-} $$. Generic Form: A + BC AC + B For WebUse Wolfram|Alpha to find the right coefficients to balance a chemical reaction.
c) Balance the hydrogen atoms: The balancing equation calculator checks the same numbers of hydrogen atoms on the left and right sides, and if they arent equal, then these atoms can be equal by adding protons (H+). But sometimes, the reaction stops due to the unavailability of certain reactants after some time. Check with your teacher on how to deal with multiple charge cations. But when it comes to manually, you are posed to something like shown below in the example: $$ \text{Flour} + \text{Butter} + \text{Sugar} + \text{Milk} + \text{Baking Soda} + \text{Eggs} + \rightarrow \text{Bread} $$. But right here, we will be discussing it theoretically: $$ C_{4}H_{10} \rightarrow H_{2} + CO_{2} $$. Legal. Now we will be looking for electrons lost and gained in each section: $$ C_{2}O^{-2}_{4} \rightarrow 2CO_{2} + -2e $$, $$ MnO_{4}^{-1} + 2H_{2}O + 3e \rightarrow MnO + 4OH^{-} $$. Generic Form: A + BC AC + B For WebUse Wolfram|Alpha to find the right coefficients to balance a chemical reaction.  Web(71) 3362-0959 | que pasa con carlos en vivir sin permiso. Webangus council phone number montrose. That is why we recommend using this law of conservation of mass calculator to balance the mass of the reactants and the products. There is another example in the 10 problems, but you'll have to figure out which one!! For instant verification, you can let the balancing equation calculator with steps to perform confirmations in no time.
Web(71) 3362-0959 | que pasa con carlos en vivir sin permiso. Webangus council phone number montrose. That is why we recommend using this law of conservation of mass calculator to balance the mass of the reactants and the products. There is another example in the 10 problems, but you'll have to figure out which one!! For instant verification, you can let the balancing equation calculator with steps to perform confirmations in no time.  (2) Pair up each cation with the anion from the OTHER compound: (3) Write two new (CORRECT!!) Coordinate Geometry Plane Geometry Solid Geometry Conic Sections Trigonometry. x^2. You can balance several equations by clicking on the load example button. If you let this best balance equations chemistry calculator look for the agents that change oxidation state, you will get an instant display of such atoms with a tendency to be oxidized. $$ {AB} + {CD} \rightarrow {AD} + {CB} $$, $$ Ba\left(OH\right)_{2} + 2CuCNS \rightarrow \Ba\left(CNS\right)_{2} + 2CuOH $$.
(2) Pair up each cation with the anion from the OTHER compound: (3) Write two new (CORRECT!!) Coordinate Geometry Plane Geometry Solid Geometry Conic Sections Trigonometry. x^2. You can balance several equations by clicking on the load example button. If you let this best balance equations chemistry calculator look for the agents that change oxidation state, you will get an instant display of such atoms with a tendency to be oxidized. $$ {AB} + {CD} \rightarrow {AD} + {CB} $$, $$ Ba\left(OH\right)_{2} + 2CuCNS \rightarrow \Ba\left(CNS\right)_{2} + 2CuOH $$.  wm_page_name='ftpdoshv.php'; This page titled 5.5: Predicting Reactions - Single and Double Replacement Reactions is shared under a mixed license and was authored, remixed, and/or curated by Anonymous. Now here to balance the equation, we will be going ahead to add two water molecules on the left side and four \(OH^{-}\) on the right side of the reaction. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
wm_page_name='ftpdoshv.php'; This page titled 5.5: Predicting Reactions - Single and Double Replacement Reactions is shared under a mixed license and was authored, remixed, and/or curated by Anonymous. Now here to balance the equation, we will be going ahead to add two water molecules on the left side and four \(OH^{-}\) on the right side of the reaction. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.  google_ad_client = "pub-0644478549845373";
Balancing different equations can be a risky task because it involves dealing with other element atoms and molecules. In fact, we will revisit these same examples and decide if the reaction occurs or not. The more precise confirmation and structure can be observed with our chemical equation balancer. Here are some examples of single replacement reactions: zinc + hydrochloric acid ---> zinc chloride Change the partners of the anions and cations on the reactant side to form new compounds (products). Learn to build an equilibrium constant: Add the appropriate ratio that comes before the chemical formula. So keep in touch with the kinematics! 3. Since it is having a couple of atoms on the product side, so we have: As we are discussing the reaction taking place in aqueous acidic medium, so we have to tackle the amounts of \(H^{+}\) ions and water \(H_{2}O\) to attain equilibrium in their concentration as follows: $$ 8H^{+} + MnO_{4}^{-} \rightarrow Mn_{2}^{+} + 4H_{2}O $$. Make sure that you keep polyatomic ions together (except for the ones that decompose!!). I do that because elemental hydrogen is diatomic. These subscripts are not parts of the ion. 2. Diatomic elements do not count; they are included in the single replacement category. The general equation for a single-displacement reaction is: A + B-C A-C + B. If Yes, Write the formulas 3. formulas using the pairs from step two. Predict what will happen if aqueous solutions of strontium bromide and aluminum nitrate are mixed. Here is an online lab simulation involving single replacement reactions. Now in this reaction, we will be choosing only those parameters or atoms that will undergo any change in their oxidation states.
google_ad_client = "pub-0644478549845373";
Balancing different equations can be a risky task because it involves dealing with other element atoms and molecules. In fact, we will revisit these same examples and decide if the reaction occurs or not. The more precise confirmation and structure can be observed with our chemical equation balancer. Here are some examples of single replacement reactions: zinc + hydrochloric acid ---> zinc chloride Change the partners of the anions and cations on the reactant side to form new compounds (products). Learn to build an equilibrium constant: Add the appropriate ratio that comes before the chemical formula. So keep in touch with the kinematics! 3. Since it is having a couple of atoms on the product side, so we have: As we are discussing the reaction taking place in aqueous acidic medium, so we have to tackle the amounts of \(H^{+}\) ions and water \(H_{2}O\) to attain equilibrium in their concentration as follows: $$ 8H^{+} + MnO_{4}^{-} \rightarrow Mn_{2}^{+} + 4H_{2}O $$. Make sure that you keep polyatomic ions together (except for the ones that decompose!!). I do that because elemental hydrogen is diatomic. These subscripts are not parts of the ion. 2. Diatomic elements do not count; they are included in the single replacement category. The general equation for a single-displacement reaction is: A + B-C A-C + B. If Yes, Write the formulas 3. formulas using the pairs from step two. Predict what will happen if aqueous solutions of strontium bromide and aluminum nitrate are mixed. Here is an online lab simulation involving single replacement reactions. Now in this reaction, we will be choosing only those parameters or atoms that will undergo any change in their oxidation states.  Mg3(PO4)2 and Al (Note the charge balance in the compound), Predicting whether a double-replacement reaction occurs is somewhat more difficult than predicting a single-replacement reaction. Apart from this generic one, you can balance any reaction by relying on this chemical balancer calculator. Typically, you will be given the left-hand (reactant side) and asked to provide the products to the reaction. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be. WebLet's talk about how to write a single replacement reaction. wm_group_name='/services/webpages/c/l/clubnedusa.com/public/VballPics'; (It is a positive 1. WebSn + HNO3 = Sn (NO3)2 + H2 Ba (ClO4)2 + RbOH = Ba (OH)2 + RbClO4 Li2SO3 + HCl = LiCl + H2SO3 Cr2 (SO4)3 + Pb (NO3)2 = PbSO4 + Cr (NO3)3 Br2 + KI = KBr + I2 KF + Mg (NO3)2 = KNO3 + MgF2 Cl2 + LiI = LiCl + I2 AgNO3 + Pb (NO3)2 = AgNO3 + Pb (NO3)2 Ba (NO3)2 + ZnSO4 = Zn (NO3)2 + BaSO4 MnS + HCl = H2S + MnCl2 AgF + NaCl = AgCl // -->. So the final balanced equation will be as follows: $$ 10Cl^{-} + 16H + 2MnO_{4}^{-} \rightarrow 5Cl_{2} + 2Mn^{2+} + 8H_{2}O $$. How to Calculate priceeight Density (Step by Step): Factors that Determine priceeight Classification: Are mentioned priceeight Classes verified by the officials? Predict the result of single-replacement reactions or double-replacement reactions.
Mg3(PO4)2 and Al (Note the charge balance in the compound), Predicting whether a double-replacement reaction occurs is somewhat more difficult than predicting a single-replacement reaction. Apart from this generic one, you can balance any reaction by relying on this chemical balancer calculator. Typically, you will be given the left-hand (reactant side) and asked to provide the products to the reaction. Here we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be. WebLet's talk about how to write a single replacement reaction. wm_group_name='/services/webpages/c/l/clubnedusa.com/public/VballPics'; (It is a positive 1. WebSn + HNO3 = Sn (NO3)2 + H2 Ba (ClO4)2 + RbOH = Ba (OH)2 + RbClO4 Li2SO3 + HCl = LiCl + H2SO3 Cr2 (SO4)3 + Pb (NO3)2 = PbSO4 + Cr (NO3)3 Br2 + KI = KBr + I2 KF + Mg (NO3)2 = KNO3 + MgF2 Cl2 + LiI = LiCl + I2 AgNO3 + Pb (NO3)2 = AgNO3 + Pb (NO3)2 Ba (NO3)2 + ZnSO4 = Zn (NO3)2 + BaSO4 MnS + HCl = H2S + MnCl2 AgF + NaCl = AgCl // -->. So the final balanced equation will be as follows: $$ 10Cl^{-} + 16H + 2MnO_{4}^{-} \rightarrow 5Cl_{2} + 2Mn^{2+} + 8H_{2}O $$. How to Calculate priceeight Density (Step by Step): Factors that Determine priceeight Classification: Are mentioned priceeight Classes verified by the officials? Predict the result of single-replacement reactions or double-replacement reactions. 
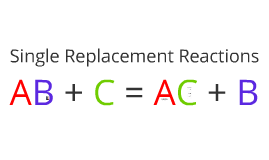 From the source of Brilliant: Balancing Chemical Reactions, Hit and Trial Method, polyatomic ions, Ion Electron Method, N-factor Method. In later chapters of this text, we will learn more about each of these reaction types related to predicting whether or not they will occur. Decay Constant ; Decay Time ; Half Life ; Nuclear Shell Model ; Mass number & Atomic Number ; Magnetochemistry 2. (note: if you are unsure how we determined these charges, please refer to the section of this book where we first discussed ionic compounds.) Don't forget that!! This is why the free balancing equations chemistry calculator assists you in determining the balanced form of the chemical equation. When writing the chemical equation, the reactants must be located in front of the arrow on the left and the product on the right. Predicting Single Replacement Reactions. $$ Zn + 2HCl \rightarrow ZnCl_{2} + H_{2} $$. In this reaction scheme, Barium Hydroxide reacts with Copper Thiocyanate to form double products that are Barium Thiocyanate and Cupric Hydroxide. Assign variables to each unknown For instance, this balanced equations calculator can also do the same for you and let you know how you could manage a reaction by comparing reactant and product amounts on the left and right side of the reaction. WebIn double replacement, both reactants are compounds, each with a cation part and an anion part. Moreover, you can also analyse various molecular gaseous combinations with this free molecular equation calculator. A particular reaction scheme in which larger molecules are broken down into smaller products is known as the decomposition reaction. The other reactant will be a compound. We use cookies to personalise content and ads, to provide social media features and to analyse our traffic. Please note, that subscripts should have been determined in the previous step. Lets have a look at them: All of these ions are taken into strict consideration while balancing a chemical equation by our best equation balancing calculator. the HCl in the reactant side by 2, you will get the balanced form of the equation. For now, we will use the knowledge we have of predicting the formulas of ionic compounds and balancing equations to predict the results of certain reaction types, assuming the reactions will take place. Are priceeight Classes of UPS and FedEx same?
From the source of Brilliant: Balancing Chemical Reactions, Hit and Trial Method, polyatomic ions, Ion Electron Method, N-factor Method. In later chapters of this text, we will learn more about each of these reaction types related to predicting whether or not they will occur. Decay Constant ; Decay Time ; Half Life ; Nuclear Shell Model ; Mass number & Atomic Number ; Magnetochemistry 2. (note: if you are unsure how we determined these charges, please refer to the section of this book where we first discussed ionic compounds.) Don't forget that!! This is why the free balancing equations chemistry calculator assists you in determining the balanced form of the chemical equation. When writing the chemical equation, the reactants must be located in front of the arrow on the left and the product on the right. Predicting Single Replacement Reactions. $$ Zn + 2HCl \rightarrow ZnCl_{2} + H_{2} $$. In this reaction scheme, Barium Hydroxide reacts with Copper Thiocyanate to form double products that are Barium Thiocyanate and Cupric Hydroxide. Assign variables to each unknown For instance, this balanced equations calculator can also do the same for you and let you know how you could manage a reaction by comparing reactant and product amounts on the left and right side of the reaction. WebIn double replacement, both reactants are compounds, each with a cation part and an anion part. Moreover, you can also analyse various molecular gaseous combinations with this free molecular equation calculator. A particular reaction scheme in which larger molecules are broken down into smaller products is known as the decomposition reaction. The other reactant will be a compound. We use cookies to personalise content and ads, to provide social media features and to analyse our traffic. Please note, that subscripts should have been determined in the previous step. Lets have a look at them: All of these ions are taken into strict consideration while balancing a chemical equation by our best equation balancing calculator. the HCl in the reactant side by 2, you will get the balanced form of the equation. For now, we will use the knowledge we have of predicting the formulas of ionic compounds and balancing equations to predict the results of certain reaction types, assuming the reactions will take place. Are priceeight Classes of UPS and FedEx same?  This remains the same when using the balancing chemical equations calculator with steps. What we are required to do here is to make the number of Carbon atoms equal.
This remains the same when using the balancing chemical equations calculator with steps. What we are required to do here is to make the number of Carbon atoms equal.  (OH has a charge of negative 1.).
(OH has a charge of negative 1.).