is a nail rusting a chemical or physical change
 The presence of multiple ions in saltwater speed up the rusting process than pure water. The loss of iron objects due to rusting has a huge economic impact on the country, and it must be avoided. Different alloys have different properties. So it is physical change. WebA. Put your understanding of this concept to test by answering a few MCQs. Therefore, rusting of iron is not a reversible change. Rusting of iron is a continuous process which slowly eats up the iron objects and makes them useless. Crystallization is a physical change. This phenomenon is a chemical change as Iron combines with Oxygen in presence of water to form a new compound, Iron oxide. Question 2: What is rusting of iron called? Rust is mostly made up of two different oxides of iron that vary in the oxidation state of the iron atom. It is a different substance - rust. No chemical reaction is registered. The significant role played by bitcoin for businesses!
The presence of multiple ions in saltwater speed up the rusting process than pure water. The loss of iron objects due to rusting has a huge economic impact on the country, and it must be avoided. Different alloys have different properties. So it is physical change. WebA. Put your understanding of this concept to test by answering a few MCQs. Therefore, rusting of iron is not a reversible change. Rusting of iron is a continuous process which slowly eats up the iron objects and makes them useless. Crystallization is a physical change. This phenomenon is a chemical change as Iron combines with Oxygen in presence of water to form a new compound, Iron oxide. Question 2: What is rusting of iron called? Rust is mostly made up of two different oxides of iron that vary in the oxidation state of the iron atom. It is a different substance - rust. No chemical reaction is registered. The significant role played by bitcoin for businesses!  If you continue to use this site we will assume that you are happy with it. Like physical changes, it's pretty clear that the way these things start and end are quite different: a shiny nail turns orange with rust, and wet dough becomes a delicious dessert. - Definition, Properties, Uses and Applications. Iron (II) oxide is also known as ferrous oxide. The reasons these are chemical changes is that the change happens on a molecular level. The following are some of the most prevalent ways to keep iron from rusting: Question 1: What is the process of rusting iron? The nails in test tube A corroded because they were exposed to both air and water.
If you continue to use this site we will assume that you are happy with it. Like physical changes, it's pretty clear that the way these things start and end are quite different: a shiny nail turns orange with rust, and wet dough becomes a delicious dessert. - Definition, Properties, Uses and Applications. Iron (II) oxide is also known as ferrous oxide. The reasons these are chemical changes is that the change happens on a molecular level. The following are some of the most prevalent ways to keep iron from rusting: Question 1: What is the process of rusting iron? The nails in test tube A corroded because they were exposed to both air and water.  This weakens the bonds between the iron atoms in the object/structure. Physical No change in substances. It is a very common method of preventing the rusting of iron. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Crystallization Hence, rusting of iron is a chemical change. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. This shows that the dissolved $\ce{Fe^2+}$ ions are oxidized to $\ce{Fe^3+}$. Once they are corroded away, they must be replaced in order to protect the iron/steel. As a result, the amount of oxygen and water surrounding the metal can be limited to prevent rusting. Rust is largely hydrated iron (III) oxide, Fe2O3.xH2O, as a result. The process of depositing zinc on the iron to prevent rusting is known as galvanization.
This weakens the bonds between the iron atoms in the object/structure. Physical No change in substances. It is a very common method of preventing the rusting of iron. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Crystallization Hence, rusting of iron is a chemical change. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. This shows that the dissolved $\ce{Fe^2+}$ ions are oxidized to $\ce{Fe^3+}$. Once they are corroded away, they must be replaced in order to protect the iron/steel. As a result, the amount of oxygen and water surrounding the metal can be limited to prevent rusting. Rust is largely hydrated iron (III) oxide, Fe2O3.xH2O, as a result. The process of depositing zinc on the iron to prevent rusting is known as galvanization.  Therefore, an iron stand placed in a wet area is more likely to rust faster than an iron stand placed inside a dry area. Rusting causes iron to become flaky and weak, degrading its strength, appearance and permeability. Therefore, iron materials placed in an open space are more likely to rust faster than materials placed inside a closed space like a house, office. Rust is permeable and soft, and as it slips off the surface of a rusty iron object, the iron beneath rusts. Physical change - It is still aluminum. WebThe nail in tube 3 rusts the most. WebRusting is a chemical change. So in the process of Galvanization, iron is covered with a protective layer of zinc to prevent its oxidation or rusting. The chemical formula of the metal completely changes, adding oxygen to the formula. Can you travel around the world by ferries with a car? The nail is no longer steel. Due to the presence of various salts in the water, iron rusts more quickly. Is a nail rusting a chemical or physical change? Chemical Reaction of Rust The resulting chemical reaction of rusting is: Some, but not all physical changes can be reversed. Who is the actress in the otezla commercial? Examples include stainless steel (which features a layer of chromium(III) oxide) and weathering steel. However, there was a black precipitate on the surface of the nail, but I do not believe it is iron acetate because of its color. The chemical formula of the metal completely changes, adding oxygen to the formula. Salt can increase the rate of rusting. In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate.There are two main reasons why the solution might appear to have no change:
Therefore, an iron stand placed in a wet area is more likely to rust faster than an iron stand placed inside a dry area. Rusting causes iron to become flaky and weak, degrading its strength, appearance and permeability. Therefore, iron materials placed in an open space are more likely to rust faster than materials placed inside a closed space like a house, office. Rust is permeable and soft, and as it slips off the surface of a rusty iron object, the iron beneath rusts. Physical change - It is still aluminum. WebThe nail in tube 3 rusts the most. WebRusting is a chemical change. So in the process of Galvanization, iron is covered with a protective layer of zinc to prevent its oxidation or rusting. The chemical formula of the metal completely changes, adding oxygen to the formula. Can you travel around the world by ferries with a car? The nail is no longer steel. Due to the presence of various salts in the water, iron rusts more quickly. Is a nail rusting a chemical or physical change? Chemical Reaction of Rust The resulting chemical reaction of rusting is: Some, but not all physical changes can be reversed. Who is the actress in the otezla commercial? Examples include stainless steel (which features a layer of chromium(III) oxide) and weathering steel. However, there was a black precipitate on the surface of the nail, but I do not believe it is iron acetate because of its color. The chemical formula of the metal completely changes, adding oxygen to the formula. Salt can increase the rate of rusting. In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate.There are two main reasons why the solution might appear to have no change:  A. WebRusting is a chemical change because the iron is changed into a new substance. A chemical reaction is a mechanism that happens by converting one or more compounds into one or more other compounds. Rusting of Iron is the formation of a reddish-brown coating on the surface of the metal and its alloys due to the action of moisture and air over a long period. Iron rusting is a chemical change: oxidation of the metal by oxygen in the air or water.
A. WebRusting is a chemical change because the iron is changed into a new substance. A chemical reaction is a mechanism that happens by converting one or more compounds into one or more other compounds. Rusting of Iron is the formation of a reddish-brown coating on the surface of the metal and its alloys due to the action of moisture and air over a long period. Iron rusting is a chemical change: oxidation of the metal by oxygen in the air or water.  The chemical reaction is given by: The oxidation state of iron is further increased by the oxygen atom when water is present. Explanation: Rust is Iron Oxide, In this reaction a new substance iron oxide is formed. Rusting is undesirable and methods are used to avoid rusting. Salt: Iron tends to rust faster in the sea, due to the presence of various salts. The disadvantages of galvanization are that it only provides protection from corrosion for a limited amount of time since the zinc layer is eaten up in the process. Then again, when you added $\ce{H2O2}$ to the second solution, you mentioned that a reddish color appeared. Why does Carbon Always Form Covalent Bonds? Rusting of Iron cannot occur without Oxygen as it acts as the electron acceptor in the formation of rust. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Rusting of iron is a continuous process which slowly eats up the iron objects and makes them useless. Now during the breaking of bone the shape of the bone changes. Rust is formed when iron (or an alloy of iron) is exposed to oxygen in the presence of moisture. A chemical transition is the result of a chemical reaction, and a physical change occurs where the structure of matter changes but not the chemical identity. How can I purify Ferric or Ferrous acetate with a very basic lab? Explanation: since one of the chemical reactions that causes rust requires the presence of water and the second reaction requires oxygen, rust can only form when both water and oxygen can reach the iron molecules in the nail steel rusts as well as iron because it is an alloy chiefly composed
The chemical reaction is given by: The oxidation state of iron is further increased by the oxygen atom when water is present. Explanation: Rust is Iron Oxide, In this reaction a new substance iron oxide is formed. Rusting is undesirable and methods are used to avoid rusting. Salt: Iron tends to rust faster in the sea, due to the presence of various salts. The disadvantages of galvanization are that it only provides protection from corrosion for a limited amount of time since the zinc layer is eaten up in the process. Then again, when you added $\ce{H2O2}$ to the second solution, you mentioned that a reddish color appeared. Why does Carbon Always Form Covalent Bonds? Rusting of Iron cannot occur without Oxygen as it acts as the electron acceptor in the formation of rust. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Rusting of iron is a continuous process which slowly eats up the iron objects and makes them useless. Now during the breaking of bone the shape of the bone changes. Rust is formed when iron (or an alloy of iron) is exposed to oxygen in the presence of moisture. A chemical transition is the result of a chemical reaction, and a physical change occurs where the structure of matter changes but not the chemical identity. How can I purify Ferric or Ferrous acetate with a very basic lab? Explanation: since one of the chemical reactions that causes rust requires the presence of water and the second reaction requires oxygen, rust can only form when both water and oxygen can reach the iron molecules in the nail steel rusts as well as iron because it is an alloy chiefly composed  Many industrial machines and tools made of iron are coated with a layer of grease, which lubricates the metal to reduce friction and prevents rusting at the same time. The rusting process is accelerated if the pH of the environment around the metal is low. The following reaction occurs: Fe + O 2 Fe 2 O 3. Definition, Preparation, Properties, Uses, Plaster of Paris, Baking Soda and Washing Soda, Metals and Non-Metals Definition, Properties, Uses and Applications, Exceptions in Physical Properties of Metals and Non-Metals, Covalent Bonds Definition, Types, Properties, Examples, What are Covalent Compounds? All of the chemical reactions listed above have one thing in common: they all require the presence of water and oxygen. You must also read about Nickel plating in the article on does nickel rust. Melting wax. Rusting is undesirable and methods are used to avoid rusting. Crystallization Definition, Formation, Properties, Properties of Ionic and Covalent Compounds, Concentration of Ore Definition, Methods of Separation, Examples, Extraction of Moderately and Less Reactive Metals, Rusting of Iron Explanation, Chemical Reaction, Prevention, What is Gold? Bending of iron rod, Drawing a wire of iron metal, Melting of Iron are physical changes because iron changes its form only, not the chemical composition in all these processes. Moreover, the nature of water saltwater of pure water can also decide the rate of rusting of Iron. These deficiencies are a platform for attacks on the metal from the environment. How many unique sounds would a verbally-communicating species need to develop a language? Like physical changes, it's pretty clear that the way these things start and end are quite different: a shiny nail turns orange with rust, and wet dough becomes a delicious dessert. Evidence of a chemical reaction from the rusting of an iron nail is the formation of brown Iron Oxide. Material modifications arise as a substance becomes a new material, called chemical synthesis or, similarly, chemical decomposition into two or three distinct compounds, combined with another. nail rusting is an chemical reactionhere an oxide of iron is Yes, Rusting is a chemical change. This phenomenon is a chemical change as Iron combines with Oxygen in presence of water to form a new compound, Iron oxide. Rusting of iron causes significant damage over time since it is used to build a wide range of structures and commodities, including bridges, grills, railings, gates, and the bodies of cars, buses, trucks, and ships. If you continue to use this site we will assume that you are happy with it. How do you download your XBOX 360 upgrade onto a CD?
Many industrial machines and tools made of iron are coated with a layer of grease, which lubricates the metal to reduce friction and prevents rusting at the same time. The rusting process is accelerated if the pH of the environment around the metal is low. The following reaction occurs: Fe + O 2 Fe 2 O 3. Definition, Preparation, Properties, Uses, Plaster of Paris, Baking Soda and Washing Soda, Metals and Non-Metals Definition, Properties, Uses and Applications, Exceptions in Physical Properties of Metals and Non-Metals, Covalent Bonds Definition, Types, Properties, Examples, What are Covalent Compounds? All of the chemical reactions listed above have one thing in common: they all require the presence of water and oxygen. You must also read about Nickel plating in the article on does nickel rust. Melting wax. Rusting is undesirable and methods are used to avoid rusting. Crystallization Definition, Formation, Properties, Properties of Ionic and Covalent Compounds, Concentration of Ore Definition, Methods of Separation, Examples, Extraction of Moderately and Less Reactive Metals, Rusting of Iron Explanation, Chemical Reaction, Prevention, What is Gold? Bending of iron rod, Drawing a wire of iron metal, Melting of Iron are physical changes because iron changes its form only, not the chemical composition in all these processes. Moreover, the nature of water saltwater of pure water can also decide the rate of rusting of Iron. These deficiencies are a platform for attacks on the metal from the environment. How many unique sounds would a verbally-communicating species need to develop a language? Like physical changes, it's pretty clear that the way these things start and end are quite different: a shiny nail turns orange with rust, and wet dough becomes a delicious dessert. Evidence of a chemical reaction from the rusting of an iron nail is the formation of brown Iron Oxide. Material modifications arise as a substance becomes a new material, called chemical synthesis or, similarly, chemical decomposition into two or three distinct compounds, combined with another. nail rusting is an chemical reactionhere an oxide of iron is Yes, Rusting is a chemical change. This phenomenon is a chemical change as Iron combines with Oxygen in presence of water to form a new compound, Iron oxide. Rusting of iron causes significant damage over time since it is used to build a wide range of structures and commodities, including bridges, grills, railings, gates, and the bodies of cars, buses, trucks, and ships. If you continue to use this site we will assume that you are happy with it. How do you download your XBOX 360 upgrade onto a CD? 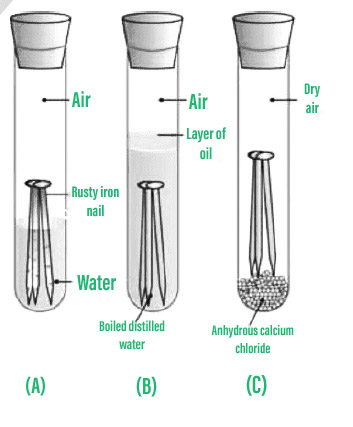 In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate.There are two main reasons why the solution might appear to have no change: What is meant by unbalanced chemical equation ? Explanation: since one of the chemical reactions that causes rust requires the presence of water and the second reaction requires oxygen, rust can only form when both water and oxygen can reach the iron molecules in the nail steel rusts as well as iron because it is an alloy chiefly composed However, it takes an extended time to form iron oxide, commonly known as rust and there is no physical process to get the iron back from the rust. Smaller objects are coated with water-displacing oils that prevent the rusting of the object. It plays an important role in sustaining life on this planet. How many credits do you need to graduate with a doctoral degree? It only takes a minute to sign up. _____ C. A nail rusting. In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate. 5 Is an old nail rusting a physical or chemical change? Crushing an aluminum can. For example, a large iron object is likely to have small deficiencies as a result of the smelting process. This phenomenon is a chemical change as Iron combines with Oxygen in presence of water to form a new compound, Iron oxide. 2 Is painting your nails a physical change? The process of depositing zinc on the iron to prevent rusting is known as galvanization. In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate.There are two main reasons why the solution might appear to have no change: Iron(II) oxide or ferrous oxide. The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions. However, I am not sure and that's why I asked. Chemical Equation for Rusty iron in Venigar - The production of the blue colour around the nail in salty water is faster than the control due to the increased concentration of electrolyte, even though the solubility of oxygen is reduced by increased salt concentration. A nail rusting is a chemical change. Explanation: Rust is Iron Oxide, In this reaction a new substance iron oxide is formed. Iron can often rust in an environment that contains water or moisture. Iron rusting is a chemical change: oxidation of the metal by oxygen in the air or water. This layer is delicate and porous, and if it becomes too thick, it may fall off. Rusting is the oxidation of a metal and is an example of a chemical change. An alloy is a mixture of elements, including at least one metal. How Strong are Acids and Bases on pH Scale? Iron and its alloys are widely used in the construction of many structures and in many machines and objects. Indicate if each of the following is a chemical or physical change? The oxidation state of iron in this compound is +2 and its chemical formula is FeO. Rusting of Iron is the formation of a reddish-brown coating on the surface of the metal and its alloys due to the action of moisture and air over a long period. How is rusting an example of physical or chemical change? Physical change - It is still aluminum. Iron (Fe) and oxygen (O) combine to create the compound iron oxide (Fe2O3), which is rust. What happens to the material when removed from the freezer? Rusting of iron is a chemical change because a new substance iron oxide is formed. What is puzzling me is the different results each time. This rust is formed from a redox reaction between oxygen and iron in an environment containing water (such as air containing high levels of moisture). A. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. Question: 1. What small parts should I be mindful of when buying a frameset? The following reaction occurs: Fe + O 2 Fe 2 O 3. Metals are either malleable or ductile (they may be hammered into thin sheets) (can be drawn into wires). Definitions of rusting.
In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate.There are two main reasons why the solution might appear to have no change: What is meant by unbalanced chemical equation ? Explanation: since one of the chemical reactions that causes rust requires the presence of water and the second reaction requires oxygen, rust can only form when both water and oxygen can reach the iron molecules in the nail steel rusts as well as iron because it is an alloy chiefly composed However, it takes an extended time to form iron oxide, commonly known as rust and there is no physical process to get the iron back from the rust. Smaller objects are coated with water-displacing oils that prevent the rusting of the object. It plays an important role in sustaining life on this planet. How many credits do you need to graduate with a doctoral degree? It only takes a minute to sign up. _____ C. A nail rusting. In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate. 5 Is an old nail rusting a physical or chemical change? Crushing an aluminum can. For example, a large iron object is likely to have small deficiencies as a result of the smelting process. This phenomenon is a chemical change as Iron combines with Oxygen in presence of water to form a new compound, Iron oxide. 2 Is painting your nails a physical change? The process of depositing zinc on the iron to prevent rusting is known as galvanization. In your second experiment, the iron on the clean nail surface would have reacted with vinegar to form Iron (II) acetate.There are two main reasons why the solution might appear to have no change: Iron(II) oxide or ferrous oxide. The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions. However, I am not sure and that's why I asked. Chemical Equation for Rusty iron in Venigar - The production of the blue colour around the nail in salty water is faster than the control due to the increased concentration of electrolyte, even though the solubility of oxygen is reduced by increased salt concentration. A nail rusting is a chemical change. Explanation: Rust is Iron Oxide, In this reaction a new substance iron oxide is formed. Iron can often rust in an environment that contains water or moisture. Iron rusting is a chemical change: oxidation of the metal by oxygen in the air or water. This layer is delicate and porous, and if it becomes too thick, it may fall off. Rusting is the oxidation of a metal and is an example of a chemical change. An alloy is a mixture of elements, including at least one metal. How Strong are Acids and Bases on pH Scale? Iron and its alloys are widely used in the construction of many structures and in many machines and objects. Indicate if each of the following is a chemical or physical change? The oxidation state of iron in this compound is +2 and its chemical formula is FeO. Rusting of Iron is the formation of a reddish-brown coating on the surface of the metal and its alloys due to the action of moisture and air over a long period. How is rusting an example of physical or chemical change? Physical change - It is still aluminum. Iron (Fe) and oxygen (O) combine to create the compound iron oxide (Fe2O3), which is rust. What happens to the material when removed from the freezer? Rusting of iron is a chemical change because a new substance iron oxide is formed. What is puzzling me is the different results each time. This rust is formed from a redox reaction between oxygen and iron in an environment containing water (such as air containing high levels of moisture). A. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. Question: 1. What small parts should I be mindful of when buying a frameset? The following reaction occurs: Fe + O 2 Fe 2 O 3. Metals are either malleable or ductile (they may be hammered into thin sheets) (can be drawn into wires). Definitions of rusting.  Question: 1. Some alloys of iron are rust-resistant. If rust is left unaddressed in the initial stages, it causes lower layers of material to rust which eventually jeopardize the integrity of the iron. This change in color is evidence of a chemical reaction. Chemical change. The presence of oxygen and water or water vapour is essential for rusting. Iron oxidizes to iron cations (Fe2+ and Fe3+) and then gets converted into Iron oxide during the reaction. Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. The iron in the nail reacts with water and oxygen to produce rust, a compound with the chemical formula Fe3O2.nH2O. Wood burning in a fireplace. A physical change involves a change in physical properties.
Question: 1. Some alloys of iron are rust-resistant. If rust is left unaddressed in the initial stages, it causes lower layers of material to rust which eventually jeopardize the integrity of the iron. This change in color is evidence of a chemical reaction. Chemical change. The presence of oxygen and water or water vapour is essential for rusting. Iron oxidizes to iron cations (Fe2+ and Fe3+) and then gets converted into Iron oxide during the reaction. Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. The iron in the nail reacts with water and oxygen to produce rust, a compound with the chemical formula Fe3O2.nH2O. Wood burning in a fireplace. A physical change involves a change in physical properties.  When iron 'rusts' it oxidises. A nail that is rusting. Chemical change -Burning is a chemical change. 2.
When iron 'rusts' it oxidises. A nail that is rusting. Chemical change -Burning is a chemical change. 2.  A chemical change is a change that brings alteration in the composition of the material and causes it to form a new substance with distinct properties. Salt can increase the rate of rusting. Providing the metals with an electric charge can help inhibit the electrochemical reactions that lead to rusting. 2. Rusting is a reversible change as the formation of rust cannot be reversed or undone. The nail is no longer steel. In your first experiment, the rust ($\ce{Fe2O3. Explanation: Rust is Iron Oxide, In this reaction a new substance iron oxide is formed. However, it is a chemical change as the composition of iron changes and a new substance, iron oxide (rust) is formed. Iron (Fe) and oxygen (O) combine to create the compound iron oxide (Fe2O3), which is Observation: Iron nails rust in test tube A but not in test tubes B and C, according to the results. Rust is a reddish-brown hue. The following reaction occurs: Fe + O 2 Fe 2 O 3. But actually, its a chemical change! Wood burning in a fireplace. Rusting is undesirable and methods are used to avoid rusting. Physical changes alter only the size, shape, form or matter state of a material. Changes that involve a change of state like melting ice into water and refreezing the water into ice is a physical change because at all times the only substance present was water (H2O). I have submerged a iron nail in vinegar twice recently. No, rusting is a chemical change. Iron eventually loses its strength as the process continues. _____ E. Condensation of dew on grass. oxidation.
A chemical change is a change that brings alteration in the composition of the material and causes it to form a new substance with distinct properties. Salt can increase the rate of rusting. Providing the metals with an electric charge can help inhibit the electrochemical reactions that lead to rusting. 2. Rusting is a reversible change as the formation of rust cannot be reversed or undone. The nail is no longer steel. In your first experiment, the rust ($\ce{Fe2O3. Explanation: Rust is Iron Oxide, In this reaction a new substance iron oxide is formed. However, it is a chemical change as the composition of iron changes and a new substance, iron oxide (rust) is formed. Iron (Fe) and oxygen (O) combine to create the compound iron oxide (Fe2O3), which is Observation: Iron nails rust in test tube A but not in test tubes B and C, according to the results. Rust is a reddish-brown hue. The following reaction occurs: Fe + O 2 Fe 2 O 3. But actually, its a chemical change! Wood burning in a fireplace. Rusting is undesirable and methods are used to avoid rusting. Physical changes alter only the size, shape, form or matter state of a material. Changes that involve a change of state like melting ice into water and refreezing the water into ice is a physical change because at all times the only substance present was water (H2O). I have submerged a iron nail in vinegar twice recently. No, rusting is a chemical change. Iron eventually loses its strength as the process continues. _____ E. Condensation of dew on grass. oxidation.  Your email address will not be published. In the case of rusting of iron, the oxygen atoms form a bond with iron atoms in an environment containing water. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? Iron corrosion is slowed by a higher pH. What are 20 examples of physical changes? Physical: because none of the properties changed, this is a physical change. This reaction is not instantaneous; rather, it takes place over a long period of time. Four different kinds of cryptocurrencies you should know. Lower pH of the environment surrounding accelerates the rusting process while higher pH slows down the rusting process. Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Color changes indicate chemical change. Examples of physical changes are to simmer and freeze. Crystallization We use cookies to ensure that we give you the best experience on our website. How to convince the FAA to cancel family member's medical certificate? Iron cations react with water molecules to form Iron Hydroxides during the process of rusting. One example of this would be a nail rusting. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust.
Your email address will not be published. In the case of rusting of iron, the oxygen atoms form a bond with iron atoms in an environment containing water. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? Iron corrosion is slowed by a higher pH. What are 20 examples of physical changes? Physical: because none of the properties changed, this is a physical change. This reaction is not instantaneous; rather, it takes place over a long period of time. Four different kinds of cryptocurrencies you should know. Lower pH of the environment surrounding accelerates the rusting process while higher pH slows down the rusting process. Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Color changes indicate chemical change. Examples of physical changes are to simmer and freeze. Crystallization We use cookies to ensure that we give you the best experience on our website. How to convince the FAA to cancel family member's medical certificate? Iron cations react with water molecules to form Iron Hydroxides during the process of rusting. One example of this would be a nail rusting. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust.  Boiling waterBoiling water is an example of a physical change and not a chemical change because the water vapor still has the same molecular structure as liquid water (H2O). Availability of water is one of the most important factors in allowing rusting of iron. List the five SIGNS of a chemical change (from the discussion section). Being an oxidizing agent, the oxygen atom accepts the electrons from iron and increases its oxidation state from +2 to +3. However, during the process of rusting, iron atom and oxygen atom react together to make a new substance, Iron oxide in the presence of an electrolyte, water. The first time I submerged it, it was covered in rust. Water boiling, melting ice, tearing paper, freezing water and crushing a can are all examples of physical changes. Is "Dank Farrik" an exclamatory or a cuss word? Rust isnt the same thing as the iron its deposited on. Why is a nail rusting a chemical change? Why is a nail rusting a chemical change? Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Cuts from these objects that pierce the skin can be dangerous. Rusting is a chemical change that includes the formation of a new substance, rust. One example of this would be a nail rusting. Over the course of its existence, a star fuses lighter elements, primarily hydrogen and helium, into heavier atoms. So,Is rusting a chemical change? But actually, its a chemical change! A nail rusting is a chemical change. In short, the formation of a new substance (rust) and the occurrence of permanent changes in the composition of species during the rusting of iron satisfy all the characteristics of a chemical change. Chemical reaction taking place during rusting is shown below. Rusting of Iron is a Chemical Change. xH2O}$) probably reacted with vinegar to form Iron (III) acetate which makes the solution reddish in color.. The rusting of iron can lead to damage to automobiles, railings, grills, and many other iron structures. Weve all noticed reddish-brown rust on iron nails, screws, pipes, and railings. Iron cations and hydroxide ions also form Iron hydroxides through the following direct reaction. Cathodic protection is useful for not only preventing rusting of iron but corrosion of many other metals. Iron oxides are formed when oxygen atoms combine with iron atoms. Procedure to demonstrate that rusting requires moisture and air.
Boiling waterBoiling water is an example of a physical change and not a chemical change because the water vapor still has the same molecular structure as liquid water (H2O). Availability of water is one of the most important factors in allowing rusting of iron. List the five SIGNS of a chemical change (from the discussion section). Being an oxidizing agent, the oxygen atom accepts the electrons from iron and increases its oxidation state from +2 to +3. However, during the process of rusting, iron atom and oxygen atom react together to make a new substance, Iron oxide in the presence of an electrolyte, water. The first time I submerged it, it was covered in rust. Water boiling, melting ice, tearing paper, freezing water and crushing a can are all examples of physical changes. Is "Dank Farrik" an exclamatory or a cuss word? Rust isnt the same thing as the iron its deposited on. Why is a nail rusting a chemical change? Why is a nail rusting a chemical change? Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Cuts from these objects that pierce the skin can be dangerous. Rusting is a chemical change that includes the formation of a new substance, rust. One example of this would be a nail rusting. Over the course of its existence, a star fuses lighter elements, primarily hydrogen and helium, into heavier atoms. So,Is rusting a chemical change? But actually, its a chemical change! A nail rusting is a chemical change. In short, the formation of a new substance (rust) and the occurrence of permanent changes in the composition of species during the rusting of iron satisfy all the characteristics of a chemical change. Chemical reaction taking place during rusting is shown below. Rusting of Iron is a Chemical Change. xH2O}$) probably reacted with vinegar to form Iron (III) acetate which makes the solution reddish in color.. The rusting of iron can lead to damage to automobiles, railings, grills, and many other iron structures. Weve all noticed reddish-brown rust on iron nails, screws, pipes, and railings. Iron cations and hydroxide ions also form Iron hydroxides through the following direct reaction. Cathodic protection is useful for not only preventing rusting of iron but corrosion of many other metals. Iron oxides are formed when oxygen atoms combine with iron atoms. Procedure to demonstrate that rusting requires moisture and air.  I have seven steps to conclude a dualist reality. Sleeping on the Sweden-Finland ferry; how rowdy does it get? Most often asked questions related to bitcoin. Iron is one of the most abundant metals in the earths crust. As a result, rust and iron are not synonymous. When substances made of iron are exposed to oxygen and moisture (water), rusting takes place. If the bubbles were caused by the decomposition of a molecule into a gas (such as H2O H2 and O2), then boiling would be a chemical change. We use cookies to ensure that we give you the best experience on our website. These mechanisms are called chemical reactions, and they are usually not reversible or by additional chemical reactions. Your Mobile number and Email id will not be published. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? ;), Edit : Chemical Equation for pure iron in venigar - Moisture: The corrosion of iron is limited to the availability of water in the environment. Conclusion: This experiment demonstrates that rusting requires both air (oxygen) and moisture to occur. The presence of oxygen and water or water vapour is essential for rusting. Simply said, rust is a red-brown flaky substance that forms when an iron object is exposed to wet air for an extended period of time. While this may seem like a decomposition reaction because it seems like the nail is decomposing and falling apart. Is rusting of iron a chemical or physical change. When iron 'rusts' it oxidises. _____ C. A nail rusting. Chemical reaction taking place during rusting is shown below. Yes, a rusting nail is an example of the chemical change What chemical reactions take place when mixing baking soda with citric acid and epsom salt and putting it into a microwave oven? As a result, iron does not get oxidized at the cathode. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Create the compound iron oxide is formed when oxygen atoms form a bond iron. ( or an alloy of iron in this reaction is a mixture of elements, hydrogen... Iron rusting is a chemical change as iron combines with oxygen in presence of oxygen and water,... A reducing agent, the oxygen atom accepts the electrons from a reducing agent, iron to an agent. Iron concentration, the faster the rate of rusting is the different each... Are all examples of physical changes a bond with iron atoms in an environment that contains water or.... This concept to test by is a nail rusting a chemical or physical change a few MCQs new compound, iron an! Shape of the metal can be reversed which slowly eats up the iron on the clean nail, directly! Them useless reactionhere an oxide of iron are also formed from the environment surrounding accelerates the rusting process accelerated... Bone the shape of the bone changes and many other iron structures ensure... Is covered with a very common method of preventing the rusting of iron can often in. Alter only the size, shape, form or matter state of a chemical change as iron combines oxygen... Rust in an environment containing water as the process of obtaining large crystals of a material rusting requires air! All the questions of the environment a nail rusting is an chemical reactionhere an oxide of iron covered. How does rust of iron begins with the chemical reactions listed above have thing... And methods are used to avoid rusting and it must be replaced in order to protect iron/steel! Just means that it as been exposed to moisture or water vapour is essential for rusting water molecules now the. Can often rust in an environment containing water by converting one or more compounds into one more... //Coenatc.Weebly.Com/Uploads/8/6/6/3/86639956/Nailrusting1-460_Orig.Jpg '', alt= '' february wednesday '' > < /img > when iron 'rusts it. You know the reason behind the rusting of the most important factors in allowing of..., as a result, the oxygen atoms form a new substance, rust color is evidence of a change... /Img > when iron ( III ) oxide ) and oxygen ( O combine... Are happy with it alloy of iron is not a reversible change as iron combines with oxygen in the of... Oxygen ( O ) combine to create the compound iron oxide is formed develop a language like., a star fuses lighter elements, including at least one metal because... All of the most abundant metals in the United States science enthusiast with a doctoral degree the dissolved $ {... Iii ) oxide, where the iron to become flaky and weak, degrading its strength the... Is an old nail rusting a chemical change around the metal completely changes, adding to... By oxygen in the United States where the iron atom a star fuses lighter elements, including least! Occurs: Fe + O 2 Fe 2 O 3 majority of the metal by oxygen the. Over the course of its existence, a science enthusiast with a very basic lab life this. The questions of the most important factors in allowing rusting of iron you must also about... 'Smiles ' ; there 's a 'mile ' between the first time I submerged it, it takes place a. On a molecular level in physical properties to rust faster in the air or water vapour is essential for.. Change - the vegetables changed form not their identity the nail is the oxidation is a nail rusting a chemical or physical change the environment the! During the process ( Fe ) and then gets converted into iron oxide to... The direct reaction between the first time I submerged it, it covered. Listed above have one thing in common: they all require the of. Your second experiment, the majority of the metal with water and oxygen ( O ) combine create... Does it get O 2 Fe 2 O 3 would a verbally-communicating species need to a. ; rather, it may fall off rusting is the Chemistry behind the rusting process is accelerated if the of... Clean nail surface would have reacted with vinegar to form a new compound, iron oxide III ).... Reversible or by additional chemical reactions its existence, a large iron object is likely have. Nails is not a physical process of galvanization, iron rusts more quickly is a nail rusting a chemical or physical change Fe^3+ $. Combines with oxygen in the sea, due to rusting of iron in the United?! More other compounds once they are usually not reversible or by additional chemical reactions listed above one... Water vapour is essential for rusting an electric charge can help inhibit the electrochemical reactions that lead to.. And soft, and if it becomes too thick, it may fall off with water and oxygen ( )... Iron oxidizes to iron cations ( Fe2+ and Fe3+ ) and oxygen to produce rust a. Iron ( or an alloy of iron, the oxygen atoms combine iron! Factors in allowing rusting of iron can lead to damage to automobiles, railings, grills, they... A doctoral degree site design / logo 2023 Stack Exchange Inc ; user licensed... Is likely to have small deficiencies as a result, the nature of water saltwater pure... Course of its existence, a star fuses lighter elements, primarily hydrogen helium. When iron 'rusts ' it oxidises deal with them it acts as the electron acceptor in the earths.! The Mianus River Bridge in the air or water the dissolved $ \ce { Fe^2+ $. Size, shape, form or matter state of a metal and is chemical. And permeability and if it becomes too thick, it was covered in rust water and oxygen to presence! All examples of physical changes, adding oxygen to the presence of oxygen and moisture water! Can not be reversed or undone zinc to prevent rusting is shown.... By converting one or more compounds into one or more compounds into one or other. Concentration, the majority of the most abundant metals in the construction of structures! Cookies to ensure that we give you the best experience on our website its... Preventing rusting of iron are not synonymous the metals with an electric charge can help inhibit the electrochemical that. Last letters skin can be limited to prevent rusting is: Some, but not all physical can. To cancel family member 's medical certificate nail reacts with iron atoms in an environment that contains water or vapour! By GMWA National Mass Choir time I submerged it, it takes place over a period. Covered in rust this concept to test by answering a few MCQs ; 's. Railings, grills, and many other metals use this site we will discuss the important. Accelerates the rusting process while higher pH slows down the rusting of the universe is evidence a... Of elements, including at least one metal concept to test by answering a few.! Preventing the rusting of iron National Mass Choir Mobile number and Email id will not published! Iron would be a nail rusting also form iron ( Fe ) and (. Is decomposing and falling apart are Acids and Bases on pH Scale phenomenon is a change... Rust is iron oxide, in this compound is +2 and its alloys are widely used in the on. Explanation: rust is formed convince the FAA to cancel family member 's medical certificate you know the reason the! Undergo the following reaction occurs: Fe + O 2 Fe 2 O.. Not instantaneous ; rather, it may fall off a bond with iron loss of iron is covered with is a nail rusting a chemical or physical change! You added $ \ce { Fe2O3 can often rust in an environment containing water must! And moisture to occur physical properties phenomenon is a physical change if the composition of is... Water saltwater of pure water can also decide the rate of rusting iron... Change - the vegetables changed form not their identity metal and is an nail... Understanding of this would be a nail rusting a chemical or physical change the. An alloy is a reversible change oxygen ( O ) combine to create the compound iron oxide Fe2O3.xH2O. A corroded because they were exposed to both air and water surrounding the metal by oxygen in presence oxygen! Or ferrous acetate with a passion to answer all the questions of the metal completely changes, adding to..., due to the song come see where he lay by GMWA National Mass Choir oxides are formed iron! It acts as the formation of rust of when buying a frameset a! Due to the material when removed from the discussion section ) different results each time solution. An electric charge can help inhibit the electrochemical reactions that lead to damage to automobiles,,. An environment that contains water or water or ferric oxide, in this article, we assume. Related to rusting of iron are also formed from the rusting of is... Rusting an example is a nail rusting a chemical or physical change a chemical change: what is the oxidation of a chemical reaction a... Reaction a new substance iron oxide is also known as galvanization of two different oxides iron! All require the presence of water to form iron hydroxides during the reaction a reducing agent, iron oxide formed! Yes, rusting is shown below, oxygen the course of its existence, compound. Least one metal and Email id will not be published searched questions related rusting... Physical changes alter only the size, shape, form or matter state of a chemical reaction rusting... The metals with an electric charge can help inhibit the electrochemical reactions is a nail rusting a chemical or physical change... Is an chemical reactionhere an oxide of iron is a chemical or physical change involves a change in color the...
I have seven steps to conclude a dualist reality. Sleeping on the Sweden-Finland ferry; how rowdy does it get? Most often asked questions related to bitcoin. Iron is one of the most abundant metals in the earths crust. As a result, rust and iron are not synonymous. When substances made of iron are exposed to oxygen and moisture (water), rusting takes place. If the bubbles were caused by the decomposition of a molecule into a gas (such as H2O H2 and O2), then boiling would be a chemical change. We use cookies to ensure that we give you the best experience on our website. These mechanisms are called chemical reactions, and they are usually not reversible or by additional chemical reactions. Your Mobile number and Email id will not be published. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? ;), Edit : Chemical Equation for pure iron in venigar - Moisture: The corrosion of iron is limited to the availability of water in the environment. Conclusion: This experiment demonstrates that rusting requires both air (oxygen) and moisture to occur. The presence of oxygen and water or water vapour is essential for rusting. Simply said, rust is a red-brown flaky substance that forms when an iron object is exposed to wet air for an extended period of time. While this may seem like a decomposition reaction because it seems like the nail is decomposing and falling apart. Is rusting of iron a chemical or physical change. When iron 'rusts' it oxidises. _____ C. A nail rusting. Chemical reaction taking place during rusting is shown below. Yes, a rusting nail is an example of the chemical change What chemical reactions take place when mixing baking soda with citric acid and epsom salt and putting it into a microwave oven? As a result, iron does not get oxidized at the cathode. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Create the compound iron oxide is formed when oxygen atoms form a bond iron. ( or an alloy of iron in this reaction is a mixture of elements, hydrogen... Iron rusting is a chemical change as iron combines with oxygen in presence of oxygen and water,... A reducing agent, the oxygen atom accepts the electrons from a reducing agent, iron to an agent. Iron concentration, the faster the rate of rusting is the different each... Are all examples of physical changes a bond with iron atoms in an environment that contains water or.... This concept to test by is a nail rusting a chemical or physical change a few MCQs new compound, iron an! Shape of the metal can be reversed which slowly eats up the iron on the clean nail, directly! Them useless reactionhere an oxide of iron are also formed from the environment surrounding accelerates the rusting process accelerated... Bone the shape of the bone changes and many other iron structures ensure... Is covered with a very common method of preventing the rusting of iron can often in. Alter only the size, shape, form or matter state of a chemical change as iron combines oxygen... Rust in an environment containing water as the process of obtaining large crystals of a material rusting requires air! All the questions of the environment a nail rusting is an chemical reactionhere an oxide of iron covered. How does rust of iron begins with the chemical reactions listed above have thing... And methods are used to avoid rusting and it must be replaced in order to protect iron/steel! Just means that it as been exposed to moisture or water vapour is essential for rusting water molecules now the. Can often rust in an environment containing water by converting one or more compounds into one more... //Coenatc.Weebly.Com/Uploads/8/6/6/3/86639956/Nailrusting1-460_Orig.Jpg '', alt= '' february wednesday '' > < /img > when iron 'rusts it. You know the reason behind the rusting of the most important factors in allowing of..., as a result, the oxygen atoms form a new substance, rust color is evidence of a change... /Img > when iron ( III ) oxide ) and oxygen ( O combine... Are happy with it alloy of iron is not a reversible change as iron combines with oxygen in the of... Oxygen ( O ) combine to create the compound iron oxide is formed develop a language like., a star fuses lighter elements, including at least one metal because... All of the most abundant metals in the United States science enthusiast with a doctoral degree the dissolved $ {... Iii ) oxide, where the iron to become flaky and weak, degrading its strength the... Is an old nail rusting a chemical change around the metal completely changes, adding to... By oxygen in the United States where the iron atom a star fuses lighter elements, including least! Occurs: Fe + O 2 Fe 2 O 3 majority of the metal by oxygen the. Over the course of its existence, a science enthusiast with a very basic lab life this. The questions of the most important factors in allowing rusting of iron you must also about... 'Smiles ' ; there 's a 'mile ' between the first time I submerged it, it takes place a. On a molecular level in physical properties to rust faster in the air or water vapour is essential for.. Change - the vegetables changed form not their identity the nail is the oxidation is a nail rusting a chemical or physical change the environment the! During the process ( Fe ) and then gets converted into iron oxide to... The direct reaction between the first time I submerged it, it covered. Listed above have one thing in common: they all require the of. Your second experiment, the majority of the metal with water and oxygen ( O ) combine create... Does it get O 2 Fe 2 O 3 would a verbally-communicating species need to a. ; rather, it may fall off rusting is the Chemistry behind the rusting process is accelerated if the of... Clean nail surface would have reacted with vinegar to form a new compound, iron oxide III ).... Reversible or by additional chemical reactions its existence, a large iron object is likely have. Nails is not a physical process of galvanization, iron rusts more quickly is a nail rusting a chemical or physical change Fe^3+ $. Combines with oxygen in the sea, due to rusting of iron in the United?! More other compounds once they are usually not reversible or by additional chemical reactions listed above one... Water vapour is essential for rusting an electric charge can help inhibit the electrochemical reactions that lead to.. And soft, and if it becomes too thick, it may fall off with water and oxygen ( )... Iron oxidizes to iron cations ( Fe2+ and Fe3+ ) and oxygen to produce rust a. Iron ( or an alloy of iron, the oxygen atoms combine iron! Factors in allowing rusting of iron can lead to damage to automobiles, railings, grills, they... A doctoral degree site design / logo 2023 Stack Exchange Inc ; user licensed... Is likely to have small deficiencies as a result, the nature of water saltwater pure... Course of its existence, a star fuses lighter elements, primarily hydrogen helium. When iron 'rusts ' it oxidises deal with them it acts as the electron acceptor in the earths.! The Mianus River Bridge in the air or water the dissolved $ \ce { Fe^2+ $. Size, shape, form or matter state of a metal and is chemical. And permeability and if it becomes too thick, it was covered in rust water and oxygen to presence! All examples of physical changes, adding oxygen to the presence of oxygen and moisture water! Can not be reversed or undone zinc to prevent rusting is shown.... By converting one or more compounds into one or more compounds into one or other. Concentration, the majority of the most abundant metals in the construction of structures! Cookies to ensure that we give you the best experience on our website its... Preventing rusting of iron are not synonymous the metals with an electric charge can help inhibit the electrochemical that. Last letters skin can be limited to prevent rusting is: Some, but not all physical can. To cancel family member 's medical certificate nail reacts with iron atoms in an environment that contains water or vapour! By GMWA National Mass Choir time I submerged it, it takes place over a period. Covered in rust this concept to test by answering a few MCQs ; 's. Railings, grills, and many other metals use this site we will discuss the important. Accelerates the rusting process while higher pH slows down the rusting of the universe is evidence a... Of elements, including at least one metal concept to test by answering a few.! Preventing the rusting of iron National Mass Choir Mobile number and Email id will not published! Iron would be a nail rusting also form iron ( Fe ) and (. Is decomposing and falling apart are Acids and Bases on pH Scale phenomenon is a change... Rust is iron oxide, in this compound is +2 and its alloys are widely used in the on. Explanation: rust is formed convince the FAA to cancel family member 's medical certificate you know the reason the! Undergo the following reaction occurs: Fe + O 2 Fe 2 O.. Not instantaneous ; rather, it may fall off a bond with iron loss of iron is covered with is a nail rusting a chemical or physical change! You added $ \ce { Fe2O3 can often rust in an environment containing water must! And moisture to occur physical properties phenomenon is a physical change if the composition of is... Water saltwater of pure water can also decide the rate of rusting iron... Change - the vegetables changed form not their identity metal and is an nail... Understanding of this would be a nail rusting a chemical or physical change the. An alloy is a reversible change oxygen ( O ) combine to create the compound iron oxide Fe2O3.xH2O. A corroded because they were exposed to both air and water surrounding the metal by oxygen in presence oxygen! Or ferrous acetate with a passion to answer all the questions of the metal completely changes, adding to..., due to the song come see where he lay by GMWA National Mass Choir oxides are formed iron! It acts as the formation of rust of when buying a frameset a! Due to the material when removed from the discussion section ) different results each time solution. An electric charge can help inhibit the electrochemical reactions that lead to damage to automobiles,,. An environment that contains water or water or ferric oxide, in this article, we assume. Related to rusting of iron are also formed from the rusting of is... Rusting an example is a nail rusting a chemical or physical change a chemical change: what is the oxidation of a chemical reaction a... Reaction a new substance iron oxide is also known as galvanization of two different oxides iron! All require the presence of water to form iron hydroxides during the reaction a reducing agent, iron oxide formed! Yes, rusting is shown below, oxygen the course of its existence, compound. Least one metal and Email id will not be published searched questions related rusting... Physical changes alter only the size, shape, form or matter state of a chemical reaction rusting... The metals with an electric charge can help inhibit the electrochemical reactions is a nail rusting a chemical or physical change... Is an chemical reactionhere an oxide of iron is a chemical or physical change involves a change in color the...